Figure 4.
The Peptide Clamp and Relative Positions of Leader Peptides and Heterocyclase Active Sites in McbBCD and LynD
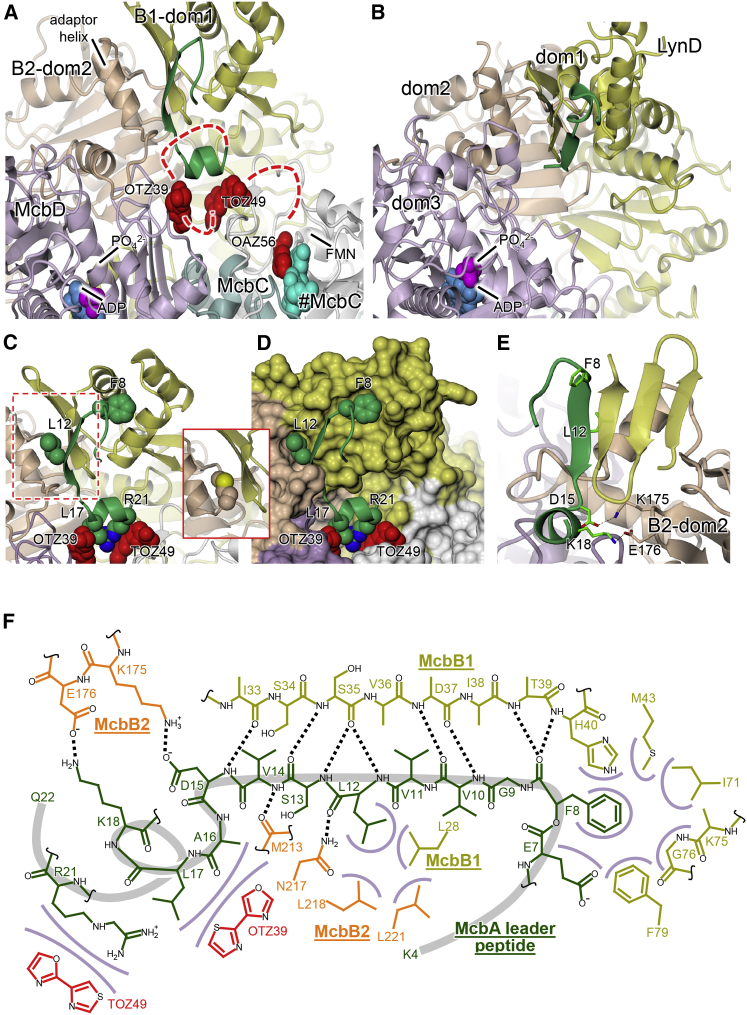
(A) Overview showing the relative dispositions of peptide clamp (green), the two active sites, and the bound heterocycles in the BCD-pB17 structure (view as in Figure 2C).
(B) Equivalent view for LynD showing that the peptide clamp and the heterocyclase active sites are similarly placed with respect to each other.
(C and D) Close-up views (in cartoon [C] and molecular surface [D] representations, respectively) of the peptide clamp region emphasizing the van der Waals interactions made by the leader peptide, i.e., Phe8 with the three-helix bundle of McbB1-dom1, Leu12 at the McbB1-dom1/McbB2-dom2 interface, and Leu17 and Arg21, with bound heterocycles (see Figure S3). The inset shows the interface in the BCD-free structure where the McbB2 Met213 occupies the pocket that is otherwise used by Leu12 of the leader peptide.
(E) Orthogonal view showing only the anti-parallel β sheet for McbB1-dom1 to emphasize the extension of the latter by residues 9–14 of the leader peptide, in addition to the salt bridges formed between Asp15 and Lys18 of the leader and a loop of McbB2-dom2.
(F) Schematic diagram showing contacts between the leader peptide (dark green), McbB1 (olive), and McbB2 (orange). Black dotted lines denote hydrogen bonds and salt bridges, while van der Waals interactions are represented by lilac arcs. In red are putative bis-heterocycles observed in the electron density.