Figure 5.
The Structure and Mechanism of the Heterocyclase McbD
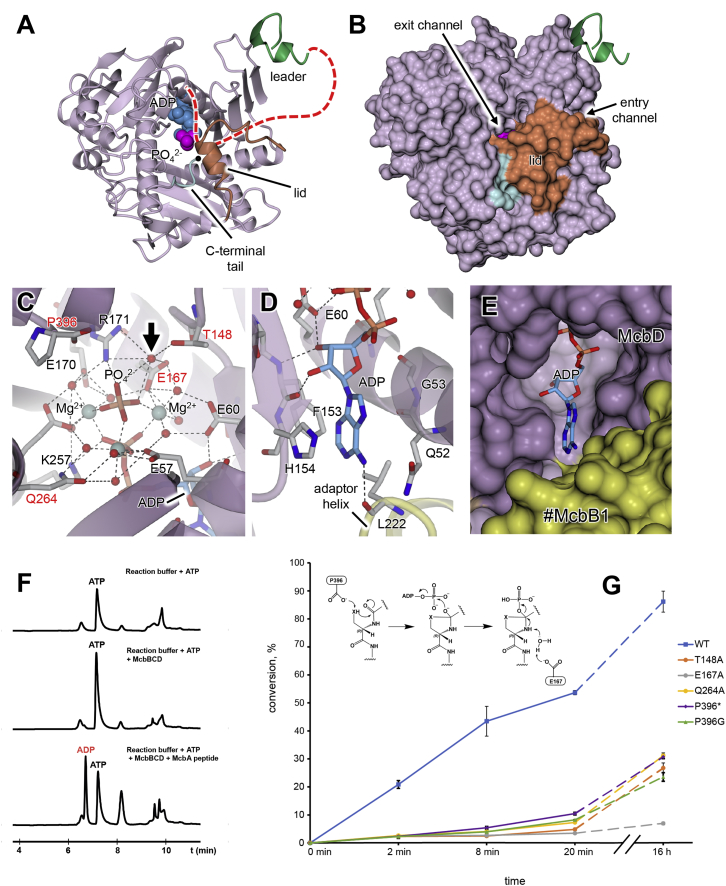
(A and B) show the isolated McbD subunit (in standard view) in cartoon (A) and molecular surface (B) representations. Also shown is the relative position of the leader peptide (green, which is somewhat in the foreground) and, as van der Waals spheres, the bound ADP (blue) and phosphate (magenta). The lid region (orange) and C-terminal tail (cyan) partially occlude the active site, although the phosphate is just visible through a pore that we designate the exit channel (see also Figure S5). A putative path for the substrate peptide extending from the end of the leader and going through the McbD active site (under the lid) is traced by the red dashed line in (A), and the Cα of the terminal Pro396 is highlighted by the black sphere.
(C) Close up of the active-site region adjacent to the phosphate ion showing side chains (in stick representation) that interact with the ADP, phosphate, and associated magnesium ions. The residues selected for mutagenesis are labeled in red. Also shown is a highly coordinated water molecule (indicated by the black arrow), for which we postulate a role in catalysis (see also Figures 5G and S6).
(D) Close up of the interactions with the ribose and base of ADP as viewed from the rear of the subunit relative to the standard view (Figure 2C), also showing the interaction with Leu222 in the adaptor helix of #McbB1.
(E) Molecular surface representation of region shown in (D), showing that nucleotide exchange is possible from this side of McbD.
(F) HPLC traces of reactions containing McbBCD and ATP. ADP is formed in the presence of a substrate peptide.
(G) A diagram of efficiency of substrate conversion by different active-center mutants along with the proposed mechanism of McbD (see also Figures S4 and S6). Briefly, we suggest that Pro396 takes the role of a general base to activate a nucleophile (X = O, S) for attack on an adjacent carbonyl leading to the postulated hemiorthoamide intermediate to be activated by ATP. Phosphate elimination initiated by a coordinated water molecule leads to the formation of an azoline. Substrate conversion efficiency was calculated similarly to (Dunbar et al., 2014) but for 9 heterocycles. Three independent reactions were analyzed for each time point, and the resultant means are presented, with errors plotted as ±1 SD.