Abstract
The present study investigated the individual and combined impact of organophosphorus pesticide chlorpyrifos (CPF) and manganese (Mn), a naturally occurring trace metal, on hepatorenal function in adult rats. The four experimental groups namely control, CPF alone (5 mg/kg), Mn alone (10 mg/kg) and the co-exposure group consisted of eight rats which were orally gavage for 14 consecutive days. Following sacrifice, the biomarkers of hepatorenal damage, antioxidant enzyme activities, myeloperoxidase (MPO) activity as well as levels of nitric oxide, reactive oxygen and nitrogen (RONS) species and lipid peroxidation (LPO) were analysed spectrophotometrically. Further, the concentration of tumour necrosis factor alpha (TNF-α), interleukin-1 β (IL-1β) and caspase-3 activity were assessed using ELISA. Results showed that the CPF-induced increase in biomarkers of hepatorenal toxicity were significantly (p < 0.05) alleviated in rats co-expose to CPF and Mn. Moreover, the decrease in antioxidant status as well as the elevation in RONS and LPO were significantly assuaged in rats co-treated with CPF and Mn. In addition, CPF mediated increase in TNF-α, IL-1β and caspase-3 activity were significantly diminished in the liver and kidney of rats co-exposed to CPF and Mn. Light microscopic examination evidenced that the severity of histopathological lesions induced by CPF were alleviated in rats co-exposed to CPF and Mn. In conclusion, the results highlight that co-exposure to CPF and Mn in rats assuaged CPF-induced oxidative stress, inflammation and caspase-3 activation in the liver and kidney of the rats.
Keywords: Manganese, Chlorpyrifos, Co-exposure, Oxido-inflammation, Caspase-3, Rats
1. Introduction
Chlorpyrifos, an organophosphorus pesticide, is commonly used in agricultural and non-agriculture sectors due to its effectiveness in pest control. Although the use of CPF has been banned in some developed countries, yet CPF has been reported to be the highest selling organophosphate insecticide currently used worldwide [1]. The widespread application of CPF has been related to its cost effectiveness as well as its potential broad spectrum toxicity against insect species without adverse effect on the seed activity [2]. However, the extensive use of CPF has been demonstrated to be poisonous to non-target species including humans following direct and indirect exposure [3]. The potential routes of exposure to CPF have been shown to include consumption of CPF-contaminated food products, inhalation and dermal absorption during mixing and application [4]. Exposure to CPF is associated with several pathological conditions including neurotoxicity, endocrine disruption, reproductive toxicity, immune dysfunction and hepatorenal damage in both animals and humans [[5], [6], [7]].
Manganese (Mn) is a naturally occurring trace element [8]. It is an important metal in several physiological processes in humans, animals and plants. Manganese is nutritionally important at low concentrations but potentially toxic at high concentrations. Specifically, Mn plays a vital role in normal growth, development and cellular homeostasis [9]. It is an integral part of metalloenzymes including antioxidant enzyme manganese superoxide dismutase as well as metabolic enzymes including glutamine synthetase, pyruvate decarboxylase and arginase [10]. Humans are often exposed to Mn in the diet as well as in the environment and occupational setting. Although Mn is indispensable for the normal cellular functioning, excessive exposure to Mn reportedly elicits pathophysiological processes in animals and humans [10,11]. For instance, industrial steel workers, miners and welders are occupationally exposed to Mn whereas residents around large industries or highly-frequented traffic routes with Mn containing exhaust from methylcyclopentadienyl manganese tricarbonyl charged fuel are at a risk of excessive Mn exposure environmentally [12,13]. Overexposure to Mn has been associated with neurotoxicity, hepatotoxicity and reproductive dysfunction in animal and humans [14].
In reality, living organisms including humans are exposed to chemical mixtures rather than single xenobiotics. The consequences of exposure to these chemical mixtures are determined by the synergistic or antagonistic effects between them [15]. The influence of chemical mixtures on the cellular processes is not fully known. Indeed, toxicology of chemical mixtures is an important toxicology field because toxic effects of these mixtures are mostly not predictable. The elucidation of the chemical mixtures impact is a major public health concern and hence, a current research interest [16,17]. Exposure to xenobiotics reportedly elicits toxicity via various mechanisms including induction of oxidative and nitrosative stress, the release of soluble pro-inflammatory cytokines including TNF-α and IL-1β, as well as activation of apoptosis [16,18]. Because human exposure to pesticide-metal mixtures is possible, the impact of their co-exposure on the liver and kidney where they are metabolized and excreted respectively, is warranted.
Up till now, the effect of co-exposure to CPF and Mn on the hepatic and renal functions is lacking literature. Considering the beneficial and toxicological significance of Mn, it is reasonable to elucidate whether Mn exacerbates or alleviates hepatorenal damage induced by pesticide CPF. The present study investigated, for the first time, the impact of sub-acute co-exposure to CPF and Mn on the biomarkers of hepatorenal function, antioxidant status, inflammation and apoptosis in adult rats.
2. Materials and methods
2.1. Chemicals
Technical grade chlorpyrifos (CPF) was obtained from Milenia Agrociências S.A. Paraná, Brazil. Manganese chloride (MnCl2·4H2O; 99.99%), thiobarbituric acid (TBA), 5′, 5′-dithiobis-2-nitrobenzoic acid (DTNB), 1-chloro-2,4-dinitrobenzene (CDNB), hydrogen peroxide (H2O2), glutathione (GSH) and epinephrine were procured from Sigma Chemical Co. (St Louis, MO, USA). ELISA kits for the assessment of tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β) and caspase 3 (CASP3) activity were purchased from Elabscience Biotechnology Company, Beijing, China. All other chemicals were of analytical grade and were obtained from the British Drug Houses (Poole, Dorset, UK).
2.2. Animal model and care
Thirty-two adult male Wistar rats (8 weeks old, 164 ± 7 g) were obtained from the Experimental Animal Unit, Faculty of Veterinary Medicine, University of Ibadan. The rats were kept in plastic cages located in a well-ventilated rat house, provided rat food and water ad libitum. The rats were subjected to natural photoperiod of 12-hr light: 12-hr dark and adequately cared for according to the conditions stated in the ‘Guide for the Care and Use of Laboratory Animals’ published by the National Institute of Health. Moreover, the study was performed in line with the US NAS guidelines and authorization by the University of Ibadan Ethical Committee.
2.3. Experimental design
Subsequent to one week of acclimatization, the rats were randomly allotted to four groups of eight rats each and were orally treated for 14 consecutive days as follows.
Control group: The rats were administered 2 ml/kg of corn.
CPF alone group: The rats were administered 5 mg/kg of CPF alone.
Mn alone group: The rats were administered 10 mg/kg of Mn alone.
CPF and Mn group: The rats were co-administered CPF (5 mg/kg) and Mn (10 mg/kg).
The doses of CPF and Mn used in the present investigation were chosen from our pilot studies and previously published data [19]. The CPF alone group was treated with the same volumes of solvents as used for the treatment in the CPF and Mn group. The final weights of the rats were taken before the collection of blood from retro-orbital venous plexus into plain tubes and sacrifice by cervical dislocation on day 15. The serum samples were subsequently obtained by centrifugation of the clotted blood at 3000 g for 10 min. The serum samples were kept frozen at −20 °C until use for the analysis of liver and kidney function markers.
2.4. Assay of liver and kidney function indices
Analysis of serum activities of aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP) and lactate dehydrogenase (LDH) as well as creatinine and urea levels were performed using available commercial kits from Randox Laboratories Limited (UK).
2.5. Assessment of oxidative stress indices
The liver and kidney samples of the experimental rats were homogenized in 50 mM Tris–HCl buffer (pH 7.4) containing 1.15% potassium chloride. The tissue homogenates were then centrifuged at 12,000 g for 15 min at 4 °C to obtain the supernatant which was used for the assessment of oxidative stress, inflammation and apoptotic biomarkers. Hepatic and renal protein concentration was assessed according to the method described by Bradford [20]. Superoxide dismutase (SOD) activity was assessed in line with the method described by Misra and Fridovich [21]. Activity of catalase (CAT) was assessed according to the method described by Clairborne [22]. Activity of glutathione-S-transferase (GST) was measured in line with the method of Habig et al [23] whereas Glutathione peroxidase (GPx) activity was assessed according to established method [24]. Glutathione (GSH) level was assessed according to the method described by Jollow et al. [25] whereas lipid peroxidation (LPO) was assayed as malondialdehyde (MDA) in line with the established method [26].
2.6. Reactive oxygen and nitrogen species (RONS) detection
Hepatic and renal RONS production was quantified according to established protocol which is based on the RONS-dependent oxidation of 2′,7′-dichlorodihydrofluorescin diacetate (DCFH-DA) to DCF [27,28]. Briefly, the reaction mixture consisted of 10 μL of the sample, 150 μL of 0.1 M potassium phosphate buffer (pH 7.4), 35 μL of distilled water and 5 μL of DCFH-DA (200 μM, final concentration 5 μM). The fluorescence emission of DCF resulting from DCFHDA oxidation was analyzed for 10 min (30 s intervals) at 488 nm excitation and 525 nm emission wavelengths using a SpectraMax plate reader (Molecular Devices, CA, USA). The rate of DCF formation was expressed in percentage of control group.
2.7. Assay of pro-inflammatory biomarkers and caspase 3 activity
Hepatic and renal nitric oxide (NO) level was assessed using Griess reagent according to established protocol [29]. Briefly, the reaction mixture consisting of equal volume of sample and Griess reagent was incubated for 15 min before the absorbance was evaluated at 540 nm. The level of NO extrapolated from the standard curve and then expressed as Units/mg protein. Moreover, myeloperoxidase (MPO) activity was evaluated according to the method described by Granell [30]. Additionally, TNF-α and IL-1β concentrations as well as caspase-3 activity was evaluated using commercially available ELISA Kits (Elabscience Biotechnology Company, Beijing, China) with the aid of a SpectraMax plate reader (Molecular Devices, CA, USA) as stated in the manufacturer's manual.
2.8. Histopathological examination of liver and kidney
Liver and kidney samples were fixed using 10% phosphate buffered formalin (PBF) for three days. The samples were embedded in paraffin after dehydration procedures. Tissue sections of 4–5 μm were cut using a microtome before staining with hematoxylin and eosin (H & E). The tissue histology was examined under a light microscope and the histopathological aberrations scored by pathologists who were blinded to the treatment groups.
2.9. Statistical analysis
Results were analyzed using the One-way analysis of variance (ANOVA) and post hoc Bonferroni’s test with the aid of GRAPHPAD PRISM 5 (GraphPad Software, La Jolla, California, USA) to ascertain significant differences in the treatment groups. P values less than 0.05 were considered to be significant.
3. Results
3.1. Mn improves body weight gain and relative organ weights in CPF-treated rats
The data on the body weight gain and the relative liver and kidney weights of rats sub-acutely treated with CPF and Mn, singly or in combination, for 14 consecutive days are shown in Table 1. Exposure to Mn alone did not cause treatment-related effect whereas administration of CPF alone significantly (p < 0.05) decreased the body weight gain but increased the relative liver and kidney weights compared with control. However, rats co-treated with CPF and Mn exhibited improvement as evident by marked increase in the body weight gain with concomitant significant reduction in the relative liver and kidney weights when compared with CPF alone group.
Table 1.
Body weight gain and relative organ weight of rats following treatment with CPF alone, Mn alone and CPF + Mn for 14 consecutive days.
| Control | CPF alone | Mn alone | CPF + Mn | |
|---|---|---|---|---|
| Body weight gain (g) | 22.85 ± 1.06 | 11.83 ± 1.11a | 20.47 ± 2.42b | 18.56 ± 1.18ab |
| Relative Kidney weight | 2.01 ± 0.28 | 2.96 ± 0.04a | 2.08 ± 0.35b | 2.28 ± 0.32ab |
| Relative Liver weight | 4.97 ± 0.19 | 6.14 ± 0.08a | 5.11 ± 0.05b | 5.79 ± 0.06ab |
CPF, 5 mg/kg Chlorpyrifos; Mn, 10 mg/kg Manganese. n = 8.Values are mean ± SD of 8 rats. a: P < 0.05 versus Control; b:P < 0.05 versus CPF alone; c:P < 0.05 versus Mn alone.
3.2. Mn alleviates biomarkers of hepatic and renal function in CPF-treated rats
The data on the hepatorenal biomarkers in rats treated with CPF alone, Mn alone or in combination are presented in Table 2. The serum activities of AST, ALT, ALP and LDH were significantly (p < 0.05) increased in rats treated with CPF alone whereas AST and LDH were markedly increased in rats treated with Mn alone. However, the marked increases in these indices of hepatotoxicity were significantly alleviated rats co-treated with CPF and Mn as evident in the reduction of serum AST, ALT, ALP and LDH. In addition, biomarkers of renal function namely serum urea and creatinine levels were significantly increased in rats treated with CPF alone whereas they were assuaged in the rats co-treated with CPF and Mn.
Table 2.
Hepatic and renal functional indices in rats following oral exposure to CPF alone, Mn alone and both CPF and Mn for 14 consecutive days.
| Control | CPF alone | Mn alone | CPF + Mn | |
|---|---|---|---|---|
| AST(U/L) | 109.27 ± 2.63 | 158.52 ± 1.96a | 125.83 ± 1.28ab | 134.65 ± 3.01ab |
| ALT(U/L) | 63.95 ± 1.44 | 108.26 ± 3.07a | 70.74 ± 2.75b | 85.02 ± 1.11abc |
| ALP(U/L) | 80.57 ± 3.17 | 121.59 ± 1.96a | 87.05 ± 3.81b | 92.37 ± 2.47ab |
| LDH(U/L) | 2.09.54 ± 0.03 | 4.59 ± 0.15a | 2.82 ± 0.64ab | 3.36 ± 0.09ab |
| Urea(mmol/L) | 7.64.11 ± 0.97 | 19.78 ± 1.02a | 9.02 ± 0.17b | 13.08 ± 0.06abc |
| Creatinine(mmol/L) | 24.13 ± 1.05 | 38.86 ± 2.64a | 26.54 ± 2.21b | 30.09 ± 1.84ab |
CPF, 5 mg/kg Chlorpyrifos; Mn, 10 mg/kg Manganese. n = 8.Values are mean ± SD of 8 rats. a: P < 0.05 versus Control; b:P < 0.05 versus CPF alone; c:P < 0.05 versus Mn alone.
3.3. Mn improves hepatic and renal antioxidant status in rats CPF-treated rats
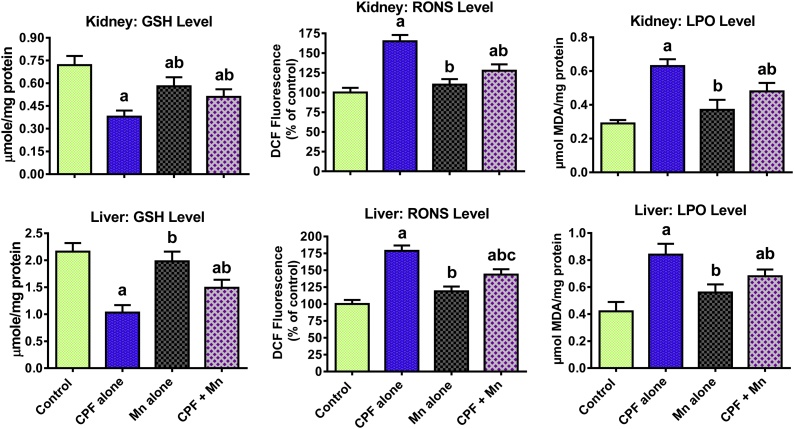
The data on the impact of sub-acute exposure to CPF and Mn, singly and in combination, on antioxidant enzymes in the liver and kidney of rats are presented on Table 3. Treatment of rats with CPF alone resulted in a significant decrease in SOD, CAT, GPx and GST activities in the liver and kidney of the treated rats when compared with the control. Exposure to Mn alone significantly decreased hepatic CAT and GST when compared with control group. However, the decrease in these antioxidant enzymes was alleviated in rats co-treated with CPF and Mn when compared with CPF alone. Further, the data on the oxidative stress indices are presented in Fig. 1. Administration of CPF alone caused a significant decrease in GSH level whereas it markedly increased RONS and LPO levels in the liver and kidney of the treated rats when compared with the control. However, the decrease in GSH level and the increase in the RONS and LPO were ameliorated in the rats co-administered with CPF and Mn when compared with CPF alone-treated rats.
Table 3.
Hepatic and renal antioxidant enzyme activities in rats following oral exposure to CPF alone, Mn alone and both CPF and Mn for 14 consecutive days.
| Control | CPF alone | Mn alone | CPF + Mn | ||
|---|---|---|---|---|---|
| SOD | Liver | 0.93 ± 0.07 | 0.45 ± 0.08a | 0.97 ± 0.04b | 0.68 ± 0.04abc |
| Kidney | 0.42 ± 0.02 | 0.17 ± 0.06a | 0.45 ± 0.03b | 0.36 ± 0.04ab | |
| CAT | Liver | 0.88 ± 0.03 | 0.46 ± 0.08a | 0.79 ± 0.01ab | 0.65 ± 0.08ab |
| Kidney | 0.69 ± 0.08 | 0.34 ± 0.06a | 0.62 ± 0.11b | 0.57 ± 0.17ab | |
| GPx | Liver | 2.88 ± 0.73 | 1.45 ± 0.37a | 2.68 ± 0.43b | 2.34 ± 0.47ab |
| Kidney | 2.76 ± 0.11 | 1.64 ± 0.14a | 2.25 ± 0.26b | 2.21 ± 0.13ab | |
| GST | Liver | 8.05 ± 0.32 | 3.96 ± 0.26a | 6.27 ± 0.54ab | 5.48 ± 0.16ab |
| Kidney | 3.53 ± 0.86 | 2.01 ± 0.12a | 3.16 ± 0.07b | 2.58 ± 0.16ab |
SOD activity (nmoles epinephrine oxidized/min/mg protein); CAT activity (μmole H2O2 consumed/min/mg protein). GPx activity (μmole of residual GSH/mg protein), GST activity (μmole CDNB–GSH complex formed/minute/mg protein). CPF, 5 mg/kg Chlorpyrifos; Mn, 10 mg/kg Manganese. n = 8. Values are mean ± SD of 8 rats. a: P < 0.05 versus Control; b:P < 0.05 versus CPF alone; c:P < 0.05 versus Mn alone.
Fig. 1.
Levels of GSH, RONS and LPO in liver and kidney of rats following oral exposure to CPF alone, Mn alone and both CPF and Mn for 14 consecutive days. CPF, 5 mg/kg Chlorpyrifos; Mn, 10 mg/kg Manganese. n = 8.Each bar represents mean ± SD of 8 rats. a: P < 0.05 versus Control; b:P < 0.05 versus CPF alone; c:P < 0.05 versus Mn alone.
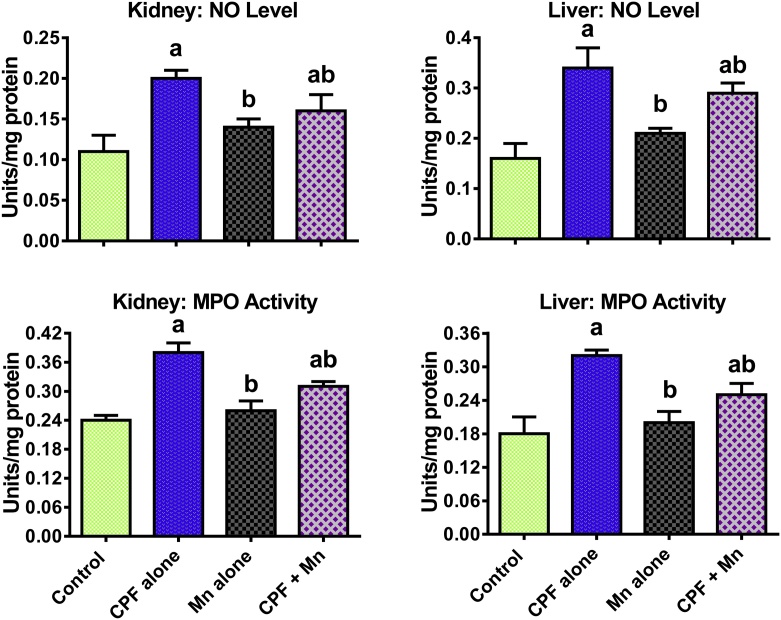
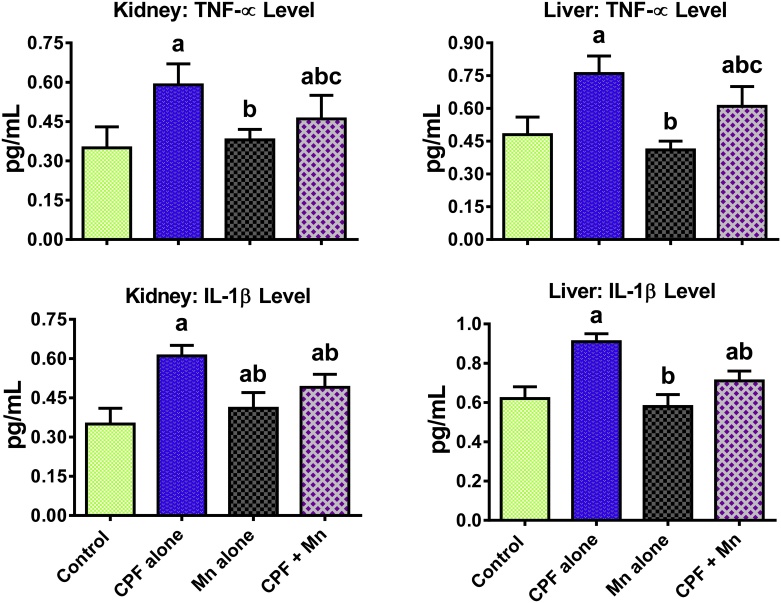
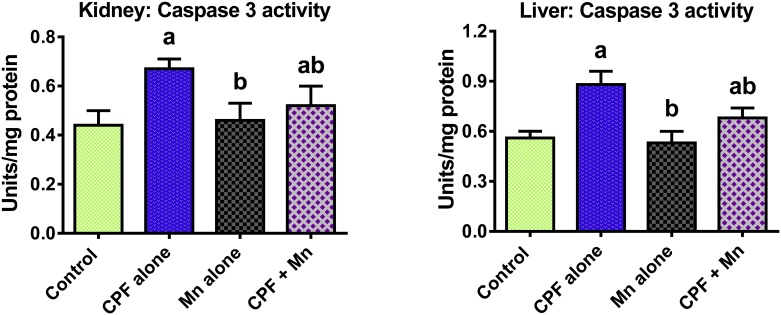
3.4. Mn suppresses pro-inflammatory biomarkers and caspase 3 activation in CPF-treated rats
The data on the influence of Mn and CPF co-exposure on biomarkers of inflammation and apoptosis are shown in Fig. 2, Fig. 3, Fig. 4. Sub-acute exposure to CPF significantly increased the hepatic and renal MPO activity as well as levels of NO, IL-1β and TNF-α in the treated rats when compared to control. Moreover, CPF alone significantly increased hepatic and renal caspase-3 activity in the treated rats when compared with control. However, the increase in these indices of inflammation and apoptosis were significantly diminished in the liver and kidney of rats co-treated with CPF and Mn when compared with CPF alone group.
Fig. 2.
Level of NO and MPO activity in liver and kidney of rats following oral exposure to CPF alone, Mn alone and both CPF and Mn for 14 consecutive days. CPF, 5 mg/kg Chlorpyrifos; Mn, 10 mg/kg Manganese. n = 8.Each bar represents mean ± SD of 8 rats. a: P < 0.05 versus Control; b:P < 0.05 versus CPF alone; c:P < 0.05 versus Mn alone.
Fig. 3.
Levels of TNF-α and IL-1β in liver and kidney of rats following oral exposure to CPF alone, Mn alone and both CPF and Mn for 14 consecutive days. CPF, 5 mg/kg Chlorpyrifos; Mn, 10 mg/kg Manganese. n = 8. Each bar represents mean ± SD of 8 rats. a: P < 0.05 versus Control; b:P < 0.05 versus CPF alone; c:P < 0.05 versus Mn alone.
Fig. 4.
Caspase-3 activity in liver and kidney of rats following oral exposure to CPF alone, Mn alone and both CPF and Mn for 14 consecutive days. CPF, 5 mg/kg Chlorpyrifos; Mn, 10 mg/kg Manganese. n = 8.Each bar represents mean ± SD of 8 rats. a: P < 0.05 versus Control; b:P < 0.05 versus CPF alone; c:P < 0.05 versus Mn alone.
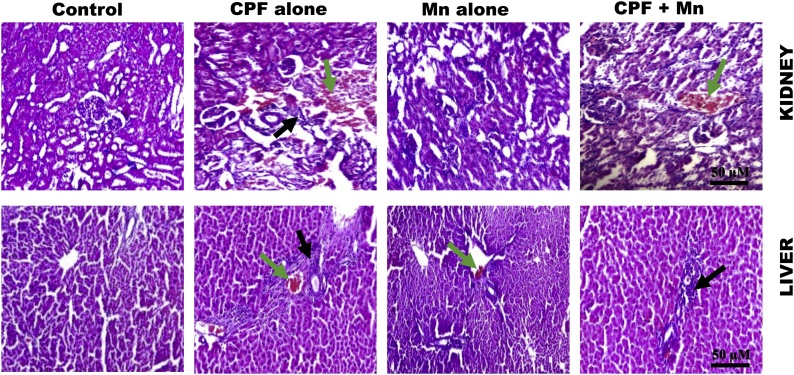
3.5. Mn ameliorates histopathological lesions in liver and kidney of CPF-treated rats
The representative photomicrographs of the liver and kidney from control and rats exposed to Mn and CPF, singly or in combination, are presented in Fig. 5. The microscopic examination of the tissues revealed normal architectures of liver and kidney of control rats. However, kidney from rats treated with CPF alone showed marked degeneration, focal area of inflammation (black arrow) and disseminated congestion of vessels (green arrow) whereas kidney from Mn alone-treated rats presented no visible lesion. Also, liver from rats treated with CPF alone showed marked degeneration, focal area of necrosis and the presence of inflammatory cells (black arrow) whereas Mn alone-treated rats showed slight congestion of vessels (green arrow). However, the histopathological lesions were alleviated in renal and hepatic cells of rats co-exposed to CPF and Mn. The details on the frequency of lesions seen in the renal and hepatic tissues of rats treated with Mn and CPF, singly or in combination are presented in Table 4.
Fig. 5.
Representative photomicrographs of liver and kidney from control, CPF alone, Mn alone and co-exposure group. Liver and kidney of control rats exhibited normal morphology. Kidney of rats treated with CPF alone showing marked degeneration, focal area of inflammation and disseminated congestion of vessels whereas Mn alone showing kidney morphology. The kidney of rats co-exposed to CPF and Mn showing mild disseminated congestion of vessels with few inflammatory cells. Liver of rats treated with CPF alone showing marked degeneration, focal area of necrosis and the presence of inflammatory cells. Mn-treated rats appear somewhat similar to control. Rats co-exposed to CPF and Mn showing the presence of few inflammatory cells.
Table 4.
Frequency of lesions detected in the kidney and liver of rats treated with CPF alone, Mn alone and both CPF and Mn for 14 consecutive days.
| Parameters | Control | CPF alone | Mn alone | CPF + Mn |
|---|---|---|---|---|
| Kidney | ||||
| Congestion of vessels | 0 | 4 | 0 | 2 |
| Focal area of inflammation | 0 | 5 | 1 | 2 |
| Fatty infiltration | 0 | 3 | 0 | 0 |
| Liver | ||||
| Focal area of necrosis | 0 | 5 | 0 | 2 |
| Large vacuoles | 0 | 2 | 0 | 0 |
| Inflammatory cells | 0 | 5 | 1 | 2 |
| Hepatic degeneration | 0 | 7 | 1 | 2 |
CPF, 5 mg/kg Chlorpyrifos; Mn, 10 mg/kg Manganese. n = 8. Values are the number of rats with observed lesions in their tissues.
4. Discussion
Human beings and animals are often exposed to mixtures of environmental contaminants including pesticides and metals [[31], [32], [33]]. Earlier investigations demonstrated that exposure to pesticides including fipronil, chlorpyrifos and deltamethrin disrupted antioxidant defense system which consequently induced oxidative stress in experimental animals whereas the adverse effects were ameliorated by the administration of exogenous antioxidants [32,34]. However, continuous low-dose dietary exposure to pesticide α-cypermethrin reportedly did not induce liver damage, oxidative stress and impaired antioxidant defense in rats [35]. The present investigation evidenced, for the first time, that co-exposure of rats to pesticide CPF and trace element Mn occasioned an alleviation in the hepatorenal damage resulting from exposure to CPF alone.
Cytosolic enzymes including ALT and AST which are localized in periportal hepatocytes are common indicators of hepatic injuries because they are often released in the blood following toxic insult [36]. Furthermore, ALP is localized in cells lining biliary duct of the liver. Thus, serum ALP activity is an index of hepatobiliary system integrity and the movement of bile into the small intestine [37] whereas serum LDH is a valuable biomarker for an early stage of acute liver injury [38,39]. In the present study, administration of CPF alone occasioned significant elevation in the serum AST, ALT, ALP and LDH level in the treated rats, thus signifying liver injury in the treated rats. Moreover, the increase in serum ALP level following administration of CPF alone connotes an obstructive event or cholestatic effect in the treated rats. The marked reduction of these biomarkers of hepatotoxicity in rats co-exposed to Mn and CPF demonstrates the antagonistic effects of Mn on CPF mediated liver damage in the treated rats.
Moreover, urea and creatinine are metabolic waste products primarily excreted in the urine. They are essential indices for assessing renal function in patients with kidney injury. Hence, the significant increase in the serum urea and creatinine levels in rats treated with CPF alone signifies renal dysfunction. Elevation in the serum creatinine level in the present study indicates deficiency in the glomerular filtration rate whereas the elevated urea level implies reduced reabsorption at the renal epithelium in CPF-treated rats. Interestingly, the obvious reduction in the serum urea and creatinine levels in rats co-administered Mn and CPF connotes the alleviating role of Mn in CPF mediated renal dysfunction in the treated rats.
Cellular antioxidant defense system consists of enzymes including SOD, CAT, GPx and GST. These endogenous antioxidants play an essential role in the direct elimination of free radicals including reactive oxygen and nitrogen species which are known to induce oxidative injury in biological components [40,41]. In the present study, administration of CPF alone caused a significant decrease in the activities of SOD, CAT, GPx and GST in both liver and kidney of the rats. The decrease in the hepatic and renal antioxidant enzymes in CPF-treated rats connotes enzyme inhibition which may result in accumulation of the cytotoxic radicals in the renal and hepatic tissues of the rats. However, the increase in the activities of these antioxidant enzymes in rats co-exposed to Mn and CPF possibly reveals the antioxidant effects of Mn against CPF mediated oxidative stress in the treated rats. It is well well-known that Mn elicits beneficial antioxidant activity devoid of the pro-oxidant side effects of other redox active metals [10,42]. Precisely, its integral role in metalloenzymes including SOD represents an enzymatic, Mn-dependent artillery for combating oxidative stress [43,44]. In addition, the hexaquo Mn2+ cation is a weak scavenger of superoxide radical but when combined with small non-proteinaceous biomolecules namely orthophosphate or carboxylates (e.g. lactate, succinate and malate) efficiently elicits antioxidant activity especially in a cellular environment with low iron [45].
Glutathione is a tripeptide with potent antioxidant activity against intracellular free radicals and peroxides [46,47]. The marked decrease in the renal and hepatic GSH level connotes its overutilization in the suppressing the free radicals concentration in the CPF-exposed rats. Further, the increase in the RONS and LPO observed in CPF-exposed rats in the present study signifies induction of oxidative damage in the liver and kidney of CPF alone-treated rats. Elevated ROS level is well known to inactivate antioxidant enzymes and consequently induces cellular oxidative damage. However, the increase in the GSH level and the marked decrease in the levels of RONS and LPO in rats co-exposed to Mn and CPF evinced the antagonistic effects of Mn on CPF mediated oxidative damage in liver and kidney of the treated rats. Thus, the mechanisms for CPF-induced oxidative stress involves suppression of antioxidant activities of SOD, CAT, GPx,GST and GSH as well as enhancement of RONS and LPO levels in liver and kidney in the treated rats. The ameliorative effect may be related to the antioxidant activity of Mn.
Besides, the present investigation revealed that CPF administration triggered a significant increase in MPO activity as well as in the levels of NO and pro-inflammatory cytokines (IL-1β and TNF-α) in the liver and kidney of rats. The heme protein MPO is often released by neutrophils and its activity is linked to induction of oxidative stress and inflammation [48]. TNF-α is a “master-regulator” of inflammatory response because it recruits immune cells at the sites of injured tissues [49]. Hence, the increase in the hepatic and renal concentrations of IL-1β and TNF-α in CPF-exposed rats evidently connotes induction of inflammation in the treated rats. Moreover, elevated TNF-α level has been shown to activate inducible nitric oxide synthase to produce NO which further combines with superoxide anion to form peroxynitrite, a noxious nitrogen species [50]. Thus, the increase in the hepatic and renal levels of NO and MPO in rats treated with CPF alonein the present study may contribute to hepatorenal damage by inducing inflammatory and nitrosative stress responses in the treated rats. Thus, CPF induced inflammation via mechanisms involving augmentation in MPO activity with concomitant increase in NO, IL-1β and TNF-α levels in liver and kidney in the treated rats. Conversely, the marked diminution in the MPO activity, levels of NO, IL-1β and TNF-α in rats co-exposed to Mn and CPF demonstrated the antagonistic effects of Mn on CPF mediated inflammatory response in liver and kidney of the treated rats.
Caspase-3 is an aspartate-specific cysteine protease well reported to regulate the apoptosis cascade [51,52]. The marked increase in the hepatic and renal caspase-3 activity in CPF alone-exposed rats connotes activation of apoptotic cell death. However, the reduction in the caspase-3 activity in rats co-exposed to CPF and Mn signifies the suppression of apoptotic cell death possibly due to antagonistic effect of Mn on CPF in the treated rats. The biochemical data of the antagonistic role of Mn in CPF-induced hepatorenal injury was corroborated by the histological findings. It is important to state that repeated exposure to CPF at 5 mg/kg/day caused neurological disturbances in the pilot studies from our laboratory. Although the treatment duration is the same, the CPF dose employed in the present study is lower than the 18 mg/kg/day of CPF previously reported to induce persistent alterations in the axonal transport [53]. Moreover, whether co-treatment with Mn reduces the absorption of CPF into the body leading to the observed beneficial effects merits further investigation.
In conclusion, sub-acute exposure to organophosphorus pesticide CPF elicits hepatorenal injury in rats via mechanisms involving induction of oxidative stress, inflammation and caspase-3 activation. The assuagement in the hepatic and renal toxicity in rats co-treated with CPF and Mn is associated with enhancement of antioxidant status, suppression of pro-inflammatory cytokines and caspase 3 activation in the exposed rats.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
This research was done without specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Burke R.D., Todd S.W., Lumsden E., Mullins R.J., Mamczarz J., Fawcett W.P., Gullapalli R.P., Randall W.R., Pereira E.F.R., Albuquerque E.X. Developmental neurotoxicity of the organophosphorus insecticide chlorpyrifos: from clinical findings to preclinical models and potential mechanisms. J. Neurochem. 2017;142:162–177. doi: 10.1111/jnc.14077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rathod A.L., Garg R.K. Chlorpyrifos poisoning and its implications in human fatal cases: A forensic perspective with reference to Indian scenario. J. Forensic Leg. Med. 2017;47:29–34. doi: 10.1016/j.jflm.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Burns C.J., McIntosh L.J., Mink P.J., Jurek A.M., Li A.A. Pesticide exposure and neurodevelopmental outcomes: review of the epidemiologic and animal studies. J. Toxicol. Environ. Health B Crit. Rev. 2013;16:127–283. doi: 10.1080/10937404.2013.783383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christensen K., Harper B., Luukinen B., Buhl K., Stone D. National Pesticide Information Center, Oregon State University Extension Services; 2009. Chlorpyrifos Technical Fact Sheet. [Google Scholar]
- 5.Noworyta-Głowacka J., Beresińska M., Bańkowski R., Wiadrowska B., Siennicka J., Ludwicki J.K. Effect of chlorpyrifos on the profile of subpopulations immunocompetent cells B, T and NK in in vivo model. Rocz. Panstw. Zakl. Hig. 2014;65:311–316. [PubMed] [Google Scholar]
- 6.Tanvir E.M., Afroz R., Chowdhury M., Gan S.H., Karim N., Islam M.N., Khalil M.I. A model of chlorpyrifos distribution and its biochemical effects on the liver and kidneys of rats. Hum. Exp. Toxicol. 2016;35:991–1004. doi: 10.1177/0960327115614384. [DOI] [PubMed] [Google Scholar]
- 7.Adedara I.A., Owoeye O., Awogbindin I.O., Ajayi B.O., Rocha J.B.T., Farombi E.O. Diphenyl diselenide abrogates brain oxidative injury and neurobehavioural deficits associated with pesticide chlorpyrifos exposure in rats. Chem. Biol. Interact. 2018;296:105–116. doi: 10.1016/j.cbi.2018.09.016. [DOI] [PubMed] [Google Scholar]
- 8.Keen C.L., Ensunsa J.L., Watson M.H., Baly D.L., Donovan S.M., Monaco M.H., Clegg M.S. Nutritional aspects of manganese from experimental studies. Neurotoxicology. 1999;20:213–223. [PubMed] [Google Scholar]
- 9.Erikson K.M., Syversen T., Aschner J., Aschner M. Interactions between excessive manganese-exposure and dietary iron deficiency in neurodegeneration. Environ. Toxicol. Pharmacol. 2005;19:415–421. doi: 10.1016/j.etap.2004.12.053. [DOI] [PubMed] [Google Scholar]
- 10.Aschner J.L., Aschner M. Nutritional aspects of manganese homeostasis. Mol. Aspects Med. 2005;26:353–362. doi: 10.1016/j.mam.2005.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfalzer A.C., Bowman A.B. Relationships between essential manganese biology and manganese toxicity in neurological disease. Curr. Environ. Health Rep. 2017;4:223–228. doi: 10.1007/s40572-017-0136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfeifer G.D., Roper J.M., Dorman D., Lynam D.R. Health and environmental testing of manganese exhaust products from use of methylcyclopentadienyl manganese tricarbonyl in gasoline. Sci. Total Environ. 2004;334-335:397–408. doi: 10.1016/j.scitotenv.2004.04.043. [DOI] [PubMed] [Google Scholar]
- 13.Rugless F., Bhattacharya A., Succop P., Dietrich K.N., Cox C., Alden J., Kuhnell P., Barnas M., Wright R., Parsons P.J., Praamsma M.L., Palmer C.D., Beidler C., Wittberg R., Haynes E.N. Childhood exposure to manganese and postural instability in children living near a ferromanganese refinery in Southeastern Ohio. Neurotoxicol. Teratol. 2014;41:71–79. doi: 10.1016/j.ntt.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang H., Wang J., Yang X., Wu F., Qi Z., Xu B., Liu W., Deng Y. Occupational manganese exposure, reproductive hormones, and semen quality in male workers: a cross-sectional study. Toxicol. Ind. Health. 2018 doi: 10.1177/0748233718810109. [DOI] [PubMed] [Google Scholar]
- 15.Groten J.P., Feron V.J., Sühnel J. Toxicology of simple and complex mixtures. Trends Pharmacol. Sci. 2001;22:316–322. doi: 10.1016/s0165-6147(00)01720-x. [DOI] [PubMed] [Google Scholar]
- 16.Wu X., Cobbina S.J., Mao G., Xu H., Zhang Z., Yang L. A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ. Sci. Pollut. Res. Int. 2016;23:8244–8259. doi: 10.1007/s11356-016-6333-x. [DOI] [PubMed] [Google Scholar]
- 17.Nys C., Van Regenmortel T., Janssen C.R., Oorts K., Smolders E., De Schamphelaere K.A.C. A framework for ecological risk assessment of metal mixtures in aquatic systems. Environ. Toxicol. Chem. 2018;37:623–642. doi: 10.1002/etc.4039. [DOI] [PubMed] [Google Scholar]
- 18.Roberts R.A., Smith R.A., Safe S., Szabo C., Tjalkens R.B., Robertson F.M. Toxicological and pathophysiological roles of reactive oxygen and nitrogen species. Toxicology. 2010;276:85–94. doi: 10.1016/j.tox.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abolaji A.O., Ojo M., Afolabi T.T., Arowoogun M.D., Nwawolor D., Farombi E.O. Protective properties of 6-gingerol-rich fraction from Zingiber officinale (Ginger) on chlorpyrifos-induced oxidative damage and inflammation in the brain, ovary and uterus of rats. Chem. Biol. Interact. 2017;270:15–23. doi: 10.1016/j.cbi.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 20.Bradford M.M. Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 21.Misra H.P., Fridovich I. The role of superoxide anion in the autooxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- 22.Clairborne A. Catalase activity. In: Greewald A.R., editor. Handbook of Methods for Oxygen Radical Research. CRC Press; Boca Raton: 1995. pp. 237–242. [Google Scholar]
- 23.Habig W.H., Pabst M.J., Jakoby W.B. Glutathione S-transferase. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 24.Rotruck J.T., Pope A.L., Ganther H.E., Swanson A.B., Hafeman D.G., Hoekstra W.G. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 25.Jollow D.J., Mitchell J.R., Zampaglione N., Gillette J.R. Bromobenzene induced liver necrosis: protective role of glutathione and evidence for 3,4 bromobenzene oxide as the hepatotoxic metabolite. Pharmacol. 1974;11:151–169. doi: 10.1159/000136485. [DOI] [PubMed] [Google Scholar]
- 26.Farombi E.O., Tahnteng J.G., Agboola A.O., Nwankwo J.O., Emerole G.O. Chemoprevention of 2-acetylaminofluorene-induced hepatotoxicity and lipid peroxidation in rats by kolaviron-a Garcinia kola seed extract. Food Chem. Toxicol. 2000;38:535–541. doi: 10.1016/s0278-6915(00)00039-9. [DOI] [PubMed] [Google Scholar]
- 27.Pérez-Severiano F., Santamaría A., Pedraza-Chaverri J., Medina-Campos O.N., Ríos C., Segovia J. Increased formation of reactive oxygen species, but no changes in glutathione peroxidase activity, in striata of mice transgenic for the Huntington’s disease mutation. Neurochem. Res. 2004;29:729–733. doi: 10.1023/b:nere.0000018843.83770.4b. [DOI] [PubMed] [Google Scholar]
- 28.Adedara I.A., Abolaji A.O., Rocha J.B., Farombi E.O. Diphenyl diselenide protects against mortality, locomotor deficits and oxidative stress in Drosophila melanogaster model of manganese-induced neurotoxicity. Neurochem. Res. 2016;41:1430–1438. doi: 10.1007/s11064-016-1852-x. [DOI] [PubMed] [Google Scholar]
- 29.Green L.C., Wagner D.A., Glogowski J., Skipper P.L., Wishnok J.S., Tannenbaum S.R. Analysis of nitrate, nitrite and [15N]nitrate in biological fluids. Anal. Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 30.Granell S., Gironella M., Bulbena O., Panés J., Mauri M., Sabater L., Aparisi L., Gelpí E., Closa D. Heparin mobilizes xanthine oxidase and induces lung inflammation in acute pancreatitis. Crit. Care Med. 2003;31:525–530. doi: 10.1097/01.CCM.0000049948.64660.06. [DOI] [PubMed] [Google Scholar]
- 31.Andrade V., Mateus M.L., Batoréu M.C., Aschner M., dos Santos A.P. Urinary delta-ALA: a potential biomarker of exposure and neurotoxic effect in rats co-treated with a mixture of lead, arsenic and manganese. Neurotoxicology. 2013;38:33–41. doi: 10.1016/j.neuro.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mossa A.H., Swelam E.S., Mohafrash S.M.M. Sub-chronic exposure to fipronil induced oxidative stress, biochemical and histopathological changes in the liver and kidney of male albino rats. Toxicol. Rep. 2015;2:775–784. doi: 10.1016/j.toxrep.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sani A., Abdullahi I.L. Evaluation of some heavy metals concentration in body fluids of metal workers in Kano metropolis. Nigeria. Toxicol Rep. 2017;4:72–76. doi: 10.1016/j.toxrep.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uchendu C., Ambali S.F., Ayo J.O., Esievo K.A.N., Umosen A.J. Erythrocyte osmotic fragility and lipid peroxidation following chronic co-exposure of rats to chlorpyrifos and deltamethrin and the beneficial effect of alpha-lipoic acid. Toxicol. Rep. 2014;1:373–378. doi: 10.1016/j.toxrep.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hongsibsong S., Stuetz W., Sus N., Prapamontol T., Grune T., Frank J. Dietary exposure to continuous small doses of α-cypermethrin in the presence or absence of dietary curcumin does not induce oxidative stress in male Wistar rats. Toxicol. Rep. 2014;1:1106–1114. doi: 10.1016/j.toxrep.2014.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaplan M.M. Laboratory tests. In: Schiff L., Schiff E.R., editors. Diseases of the Liver. 7th ed. JB Lippinocott; Philadephia: 1993. pp. 108–144. [Google Scholar]
- 37.Adedara I.A., Anao O.O., Forcados G.E., Awogbindin I.O., Agbowo A., Ola-Davies O.E., Patlolla A.K., Tchounwou P.B., Farombi E.O. Low doses of multi-walled carbon nanotubes elicit hepatotoxicity in rats with markers of oxidative stress and induction of pro-inflammatory cytokines. Biochem. Biophys. Res. Commun. 2018;503:3167–3173. doi: 10.1016/j.bbrc.2018.08.112. [DOI] [PubMed] [Google Scholar]
- 38.Kotoh K., Enjoji M., Kato M., Kohjima M., Nakamuta M., Takayanagi R. A new parameter using serum lactate dehydrogenase and alanine aminotransferase level is useful for predicting the prognosis of patients at an early stage of acute liver injury: a retrospective study. Comp. Hepatol. 2008;7:6. doi: 10.1186/1476-5926-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kotoh K., Kato M., Kohjima M., Tanaka M., Miyazaki M., Nakamura K., Enjoji M., Nakamuta M., Takayanagi R. Lactate dehydrogenase production in hepatocytes is increased at an early stage of acute liver failure. Exp. Ther. Med. 2011;2:195–199. doi: 10.3892/etm.2011.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sánchez-Valle V., Chávez-Tapia N.C., Uribe M., Méndez-Sánchez N. Role of oxidative stress and molecular changes in liver fibrosis: a review. Curr. Med. Chem. 2012;19:4850–4860. doi: 10.2174/092986712803341520. [DOI] [PubMed] [Google Scholar]
- 41.Li S., Tan H.Y., Wang N., Zhang Z.J., Lao L., Wong C.W., Feng Y. The role of oxidative stress and antioxidants in liver diseases. Int. J. Mol. Sci. 2015;16:26087–26124. doi: 10.3390/ijms161125942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coassin M., Ursini F., Bindoli A. Antioxidant effect of manganese. Arch. Biochem. Biophys. 1992;299:330–333. doi: 10.1016/0003-9861(92)90282-2. [DOI] [PubMed] [Google Scholar]
- 43.Lu L., Luo X.G., Ji C., Liu B., Yu S.X. Effect of manganese supplementation and source on carcass traits, meat quality, and lipid oxidation in broilers. J. Anim. Sci. 2007;85:812–822. doi: 10.2527/jas.2006-229. [DOI] [PubMed] [Google Scholar]
- 44.Bresciani G., da Cruz I.B., González-Gallego J. Manganese superoxide dismutase and oxidative stress modulation. Adv. Clin. Chem. 2015;68:87–130. doi: 10.1016/bs.acc.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 45.Aguirre J.D., Culotta V.C. Battles with iron: manganese in oxidative stress protection. J. Biol. Chem. 2012;287:13541–13548. doi: 10.1074/jbc.R111.312181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morris D., Khurasany M., Nguyen T., Kim J., Guilford F., Mehta R., Gray D., Saviola B., Venketaraman V. Glutathione and infection. Biochim. Biophys. Acta. 2013;1830:3329–3349. doi: 10.1016/j.bbagen.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 47.Homma T., Fujii J. Application of glutathione as anti-oxidative and anti-aging drugs. Curr. Drug Metab. 2015;16:560–571. doi: 10.2174/1389200216666151015114515. [DOI] [PubMed] [Google Scholar]
- 48.Kato Y. Neutrophil myeloperoxidase and its substrates: formation of specific markers and reactive compounds during inflammation. J. Clin. Biochem. Nutr. 2016;58:99–104. doi: 10.3164/jcbn.15-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parameswaran N., Patial S. Tumor necrosis factor-α signaling in macrophages. Crit. Rev. Eukaryot. Gene Expr. 2010;20:87–103. doi: 10.1615/critreveukargeneexpr.v20.i2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pacher P., Beckman J.S., Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhuang S., Demirs J.T., Kochevar I.E. p38 mitogen-activated protein kinase mediates bid cleavage, mitochondrial dysfunction, and caspase-3 activation during apoptosis induced by singlet oxygen but not by hydrogen peroxide. J. Biol. Chem. 2000;275:25939–25948. doi: 10.1074/jbc.M001185200. [DOI] [PubMed] [Google Scholar]
- 52.Adedara I.A., Olabiyi B.F., Ojuade T.D., Idris U.F., Onibiyo E.M., Farombi E.O. Taurine reverses sodium fluoride-mediated increase in inflammation, caspase-3 activity, and oxidative damage along the brain-pituitary-gonadal axis in male rats. Can. J. Physiol. Pharmacol. 2017;95:1019–1029. doi: 10.1139/cjpp-2016-0641. [DOI] [PubMed] [Google Scholar]
- 53.Hernandez C.M., Beck W.D., Naughton S.X., Poddar I., Adam B.L., Yanasak N., Middleton C., Terry A.V., Jr. Repeated exposure to chlorpyrifos leads to prolonged impairments of axonal transport in the living rodent brain. Neurotoxicology. 2015;47:17–26. doi: 10.1016/j.neuro.2015.01.002. [DOI] [PubMed] [Google Scholar]