Figure EV1. Hel2 and Ubc4 were involved in NGD but not NSD and NMD.
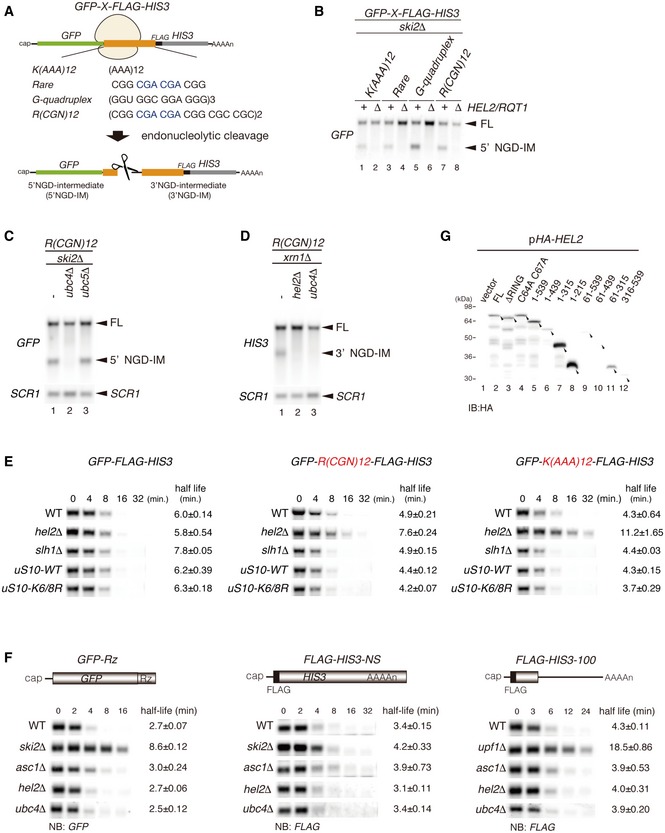
- Schematic drawing of reporter mRNAs containing various arrest‐inducing sequences. The box indicates GFP and HIS3 open reading frames (ORFs), and the black line indicates the untranslated region (UTR). Ribosome stalling takes place during translation of the indicated arrest sequences (shown in red) and induces an endonucleolytic cleavage to produce two fragments, the 5′ NGD intermediate (5′ NGD‐IM) and 3′ NGD intermediate (3′ NGD‐IM).
- Northern blot analysis showing that Hel2 is required for endonucleolytic cleavages by R(CGN)12 arrest‐inducing sequences. The GFP‐R(CGN) 12 ‐HIS3 mRNA (FL) and the 5′ NGD‐IM were detected in the indicated strains using a DIG‐labelled GFP probe. SCR1 coding for RNA in the signal recognition particle (SRP) was used as a loading control.
- Northern blot analysis showing that Ubc4 but not Ubc5 is required for endonucleolytic cleavages by R(CGN)12 arrest‐inducing sequences. The GFP‐R(CGN) 12 ‐HIS3 mRNA (FL) and the 5′ NGD‐IM were detected in the indicated strains with the DIG‐labelled GFP probe.
- Northern blotting showing that Hel2 and Ubc4 are required for endonucleolytic cleavages by R(CGN)12 arrest‐inducing sequences. The GFP‐R(CGN) 12 ‐HIS3 mRNA (FL) and the 3′ NGD‐IM were detected in the indicated strains with the DIG‐labelled HIS3 probe.
- Northern blotting showing that Hel2 reduces the half‐life of reporter mRNA containing R(CGN) 12 or K(AAA) 12 arrest sequences. The stability of GFP‐K(AAA) 12 ‐FLAG‐HIS3 and GFP‐R(CGN) 12 ‐FLAG‐HIS3 mRNAs was determined. The GFP‐K(AAA) 12 ‐FLAG‐HIS3 mRNA was stable in hel2 mutant cells than that in wild‐type cells (t 1/2 = 11.2 min for hel2Δ versus t 1/2 = 4.3 min for wild type). The GFP‐R(CGN) 12 ‐FLAG‐HIS3 mRNA was also stable in hel2 mutant cells than that in wild‐type cells (t 1/2 = 7.6 min for hel2 versus t 1/2 = 4.9 min for wild type). In contrast, the stability of GFP‐K(AAA) 12 ‐FLAG‐HIS3 mRNA was essentially the same in hel2 mutant cells (t 1/2 = 6.0 min) and in wild‐type cells (t 1/2 = 5.8 min). This stabilization of the reporter mRNAs that are substrate for NGD in hel2 mutant cells strongly supports the crucial role of Hel2 in NGD. On the other hand, mRNA stability did not change in slh1∆ and uS10‐K6/8R mutant cells.
- Hel2 is dispensable for NSD and NMD. The GFP‐Rz mRNA is a truncated poly(A) tail‐less non‐stop mRNA that is produced by self‐cleavage of hammerhead ribozyme (Rz) and efficiently degraded in the NSD pathway (Tsuboi et al, 2012). The FLAG‐HIS3‐NS mRNA is a poly(A) tail containing nonstop mRNA and subjected to NSD (van Hoof et al, 2002). The FLAG‐his3‐100 mRNA contains premature termination codon and degraded by NMD quality control (Kuroha et al, 2009). The relative levels of reporter mRNAs in the indicated mutant cells were determined using a DIG‐labeled GFP (GFP‐Rz) or 5′ DIG‐labeled‐LNA‐FLAG (FLAG‐HIS3‐NS or FLAG‐his3‐100) probes. The relative levels of each mRNA were normalized to the mRNA level in time 0, which was assigned a value of 100, and SCR RNA levels were used as a loading control for the RNA samples.
- Western blot analysis to check the expression levels of HA‐tagged Hel2 deletion mutant proteins as schematically displayed in Fig 1B using an anti‐HA antibody.
