Figure 1. SERCA2a purification and activity.
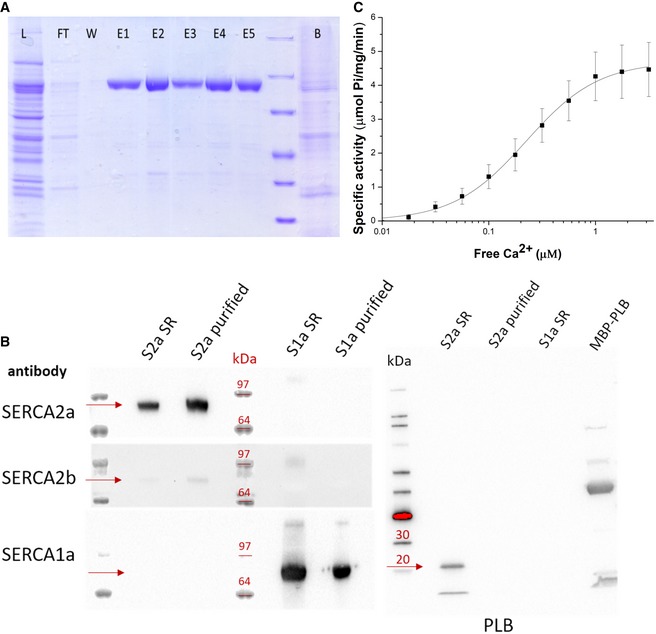
- SDS–PAGE of fractions of the SERCA2a purification procedure (L, total lysate; FT, flow‐through from Reactive Green beads; E, elution fractions; B, beads after elution).
- Immunoblot with sarcoplasmic reticulum (SR) preparations of cardiac (S2a SR) and skeletal (S1a SR) muscles, and purified SERCA2a (S2a purified) and SERCA1a (S1a purified). Detection was performed using antibodies against three SERCA isoforms 1a, 2a, and 2b, or phospholamban (PLB). PLB appears as a monomeric (5 kDa) and pentameric band (25 kDa). A fusion construct of PLB with maltose binding protein (MBP) was used as a positive control for PLB detection. The proteins of interest (e.g., SERCA or PLB) are indicated by the red arrows for each of the Western blots in the figure.
- Ca2+‐dependent ATPase activity of the purified SERCA2a. Error bars represent standard deviation; three biological replicates were analyzed. K m = 0.22 ± 0.01 μM, specific activity is 4.3 ± 0.7 μmoles/min/mg at 1 μM free Ca2+, Hill coefficient n of 1.3.
