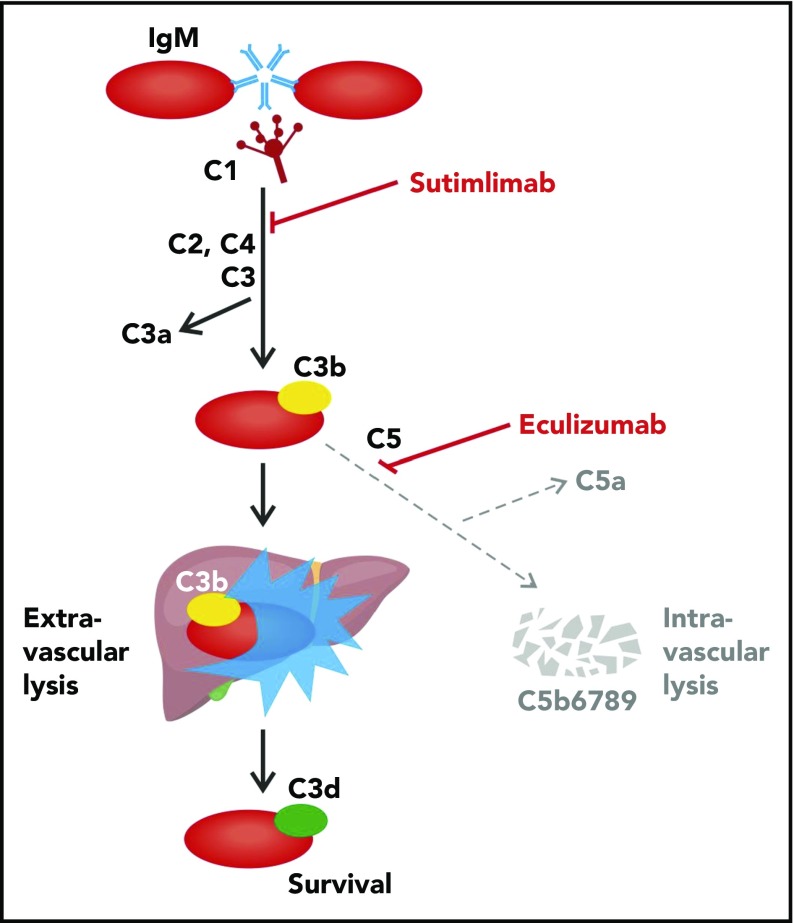
Figure 1.
Extravascular hemolysis caused by cold agglutinin–induced complement-mediated opsonization. Cold agglutinins (mostly pentameric immunoglobulin M [IgM]) agglutinate erythrocytes and fix C1, triggering the classical complement cascade and leading to C3 split product opsonization of the red blood cell. Complement-opsonized erythrocytes then travel to the liver where they are phagocytosed, a process known as extravascular hemolysis. Although complement-mediated intravascular hemolysis can occur, which requires C5 cleavage and formation of the membrane attack complex, it is generally prevented by complement regulatory proteins on the erythrocyte surface (ie, CD55 and CD59). Regardless of the hemolytic mechanism, upstream C1s blockade prevents both extravascular and intravascular hemolysis. Figure adapted and modified from Berentsen and Sundic4 and from Shi et al.12