-
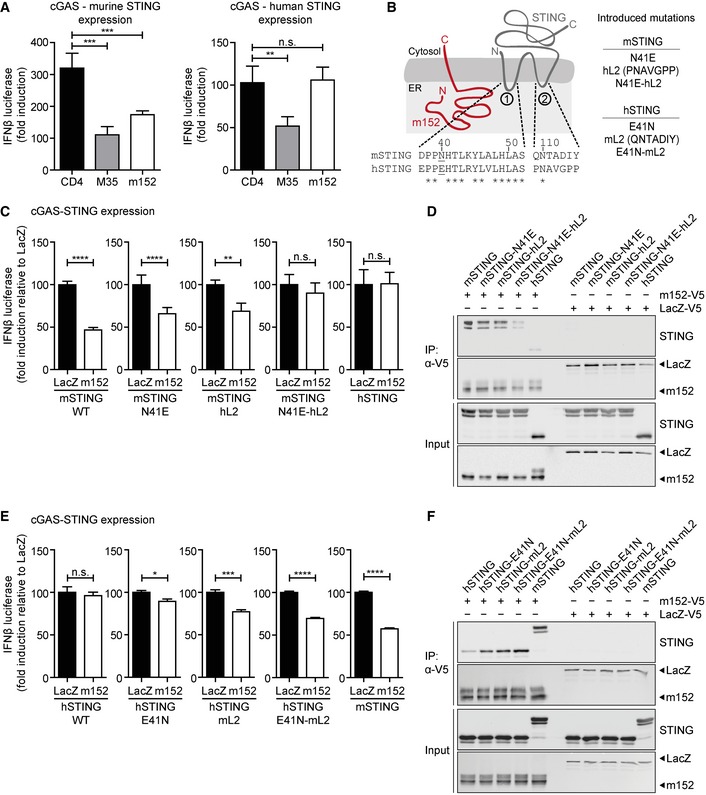
A
293T cells were co‐transfected with expression plasmids for IFNβ‐Luc, pRL‐TK, cGAS‐GFP (stimulated), or IRES‐GFP (unstimulated) and indicated expression plasmids together with murine Cherry‐STING (left panel) or human STING (right panel). Lysates were analyzed by dual‐luciferase assay. Data are combined from two out of three independent experiments and shown as mean ± SD.
-
B
Schematic representation of the predicted m152 (red) and STING (gray) topology in the ER membrane. (1) and (2) specify the ER‐luminal loop regions of STING with the sequence alignments from murine and human STING shown below. In murine STING, N41 in loop (1) was mutated to E41 and loop (2) was exchanged with loop (2) of human STING (hL2). The resultant constructs were designated mSTING N41E, mSTING hL2, and mSTING N41E‐hL2. In human STING, mutations were introduced vice versa, resulting in hSTING E41N, hSTING mL2, and hSTING E41N‐mL2.
-
C
IFNβ‐Luc, pRL‐TK, cGAS‐GFP (stimulated), or IRES‐GFP (unstimulated) and either LacZ or m152 together with the indicated murine STING mutants described in (B) were transiently expressed in 293T cells. Luciferase activity was measured as described in (A).
-
D
293T cells were co‐transfected with expression plasmids for either LacZ or m152 together with the indicated murine STING mutants described in (B). An anti‐V5 IP was performed, and samples were analyzed by IB with the indicated antibodies.
-
E, F
Luciferase assay (E) and co‐IP (F) were performed as described in (C) and (D), respectively, using the indicated human STING mutants in place of the murine STING mutants described in (B).
Data information: Data in (C) and (E) are normalized to LacZ, combined from three independent experiments and shown as mean ± SD. IB shown in (D) and (F) are representative of three independent experiments. Student's
‐test (unpaired, two‐tailed), n.s. not significant, *
< 0.0001.