Figure 2.
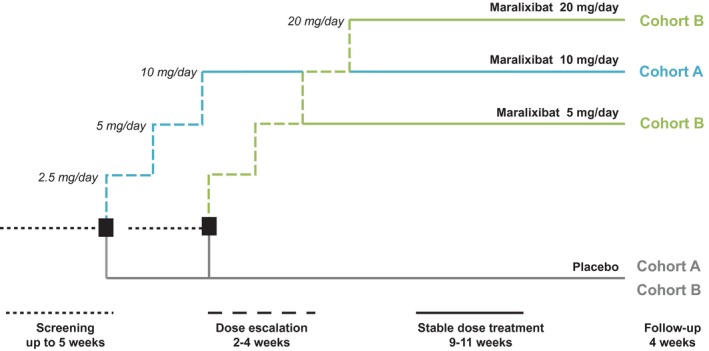
Study design. Dose selection for cohort B was guided by the tolerability of maralixibat in the first 18 patients who completed 4 weeks of treatment in cohort A; the decision was based on the number of patients who lowered their dose or suspended or stopped active treatment owing to gastrointestinal intolerance related to the maralixibat (≥5 patients, cohort B would have received 5 mg maralixibat; <5 patients, cohort B would have received 20 mg maralixibat). Clinic visits were scheduled at baseline (week 0) and weeks 2, 4, 8, and 13. Telephone contact was scheduled at weeks 1, 3, and 17 (follow‐up call).
