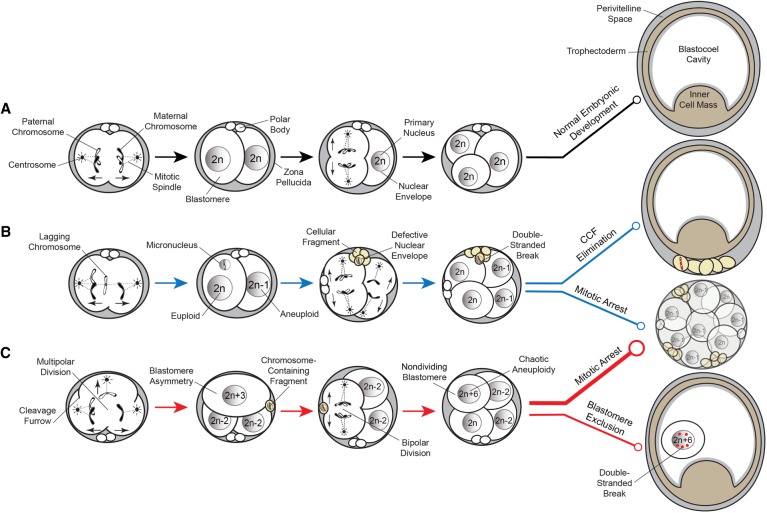
Figure 7.
Proposed model of aneuploidy generation and potential resolution in embryos. (A) Simplified model of normal embryo development, whereby a euploid zygote undergoes proper chromosome segregation with bipolar cell divisions devoid of cellular fragmentation and blastomere exclusion (black lines). (B) A euploid zygote that contains a lagging chromosome from merotelic attachments during the first mitotic division becomes encapsulated in a micronucleus. Through the process of cellular fragmentation, the chromosome is eliminated from the blastomere, where it undergoes DNA damage in the form of double-stranded breaks because of a defective nuclear envelope. This mosaic embryo is more likely to undergo mitotic arrest, but if it is able to reach the blastocyst stage, the CCF may be sequestered to the perivitelline space (blue lines). (C) Multipolar cell divisions, including a tripolar cleavage occurring at the zygote stage, may also generate CCFs as well as blastomere asymmetry and a mosaic embryo with chaotic aneuploidy. The vast majority of these embryos will eventually arrest upon embryonic genome activation when a critical number of euploid blastomeres is not achieved (thick red line). Alternatively, mosaic embryos may progress beyond the approximately eight-cell stage and aneuploid blastomeres that fail to divide during this time will sustain DNA damage and become excluded to the blastocoel cavity upon blastocyst formation (thin red line).