Abstract
Rapid and efficient digestion and absorption of dietary triglycerides and other lipids by the intestine, the packaging of those lipids into lipoprotein chylomicron (CM) particles, and their secretion via the lymphatic duct into the blood circulation are essential in maintaining whole-body lipid and energy homeostasis. Biosynthesis and assembly of CMs in enterocytes is a complex multistep process that is subject to regulation by intracellular signaling pathways as well as by hormones, nutrients, and neural factors extrinsic to the enterocyte. Dysregulation of this process has implications for health and disease, contributing to dyslipidemia and a potentially increased risk of atherosclerotic cardiovascular disease. There is increasing recognition that, besides intracellular regulation of CM assembly and secretion, regulation of postassembly pathways also plays important roles in CM secretion. This review examines recent advances in our understanding of the regulation of CM secretion in relation to mobilization of intestinal lipid stores, drawing particular attention to post-assembly regulatory mechanisms, including intracellular trafficking of triglycerides in enterocytes, CM mobilization from the lamina propria, and regulated transport of CM by intestinal lymphatics.
Keywords: Intestine, Chylomicron, Triglycerides, Lymphatics
Abbreviations used in this paper: apo, apolipoprotein; CLD, cytoplasmic lipid droplet; CM, chylomicron; CRL, Calcitonin receptor-like receptor; eNOS, endothelial nitric oxide synthase; ENS, enteric nervous system; ER, endoplasmic reticulum; FA, fatty acid; FABP, fatty acid binding protein; GLP-2, glucagon-like peptide-2; GLP-2R, glucagon-like peptide-2 receptor; LCFA, long-chain fatty acid; MG, monoglyceride; mRNA, messenger RNA; MTP, microsomal triglyceride transfer protein; NO, nitric oxide; PCTV, prechylomicron transport vesicle; Plin, perilipin; SDR39U1, short-chain dehydrogenase/reductase family 39U member 1; SMC, smooth muscle cell; SNARE, soluble NSF attachment protein receptor; STXBP5, syntaxin-binding protein 5; TG, triglyceride; VEGF-C, vascular endothelial growth factor C; VEGFR, vascular endothelial growth factor receptor; VLDL, very-low-density lipoprotein; vSNARE, vesicle soluble NSF attachment protein receptor
Summary.
Dietary fat absorption in the intestine consists of multiple steps, including absorption into enterocytes and intracellular assembly of chylomicrons, chylomicron transport and exit from the enterocytes, movement across the lamina propria, entry into lacteals, and regulated transport in intestinal lymphatics. This review draws attention to postassembly regulation of chylomicron secretion that may present opportunities for improving health.
Intestinal digestion, absorption, and secretion of dietary fats are important steps in maintaining whole-body lipid and energy homeostasis.1 Compromised lipid handling by the gut has implications for health and disease, potentially contributing to dyslipidemia and atherosclerotic cardiovascular disease. Understanding lipid processing by the gut is essential for developing novel therapeutic strategies to improve cardiac and metabolic health.2
The majority of absorbed lipids are packaged into chylomicron (CM) particles in the intestinal enterocyte, secreted into and transported through the lymphatic system to enter the blood circulation, and delivered to various tissues for storage or energy utilization. Numerous studies have elucidated the elegant cellular and molecular control of dietary fat absorption and CM biosynthesis, which has been reviewed extensively elsewhere.3, 4, 5 Recently, there has been increasing recognition that regulation of the movement of lipids and CM, retained at multiple sites in the intestinal structure, from enterocyte to circulation also contributes to the overall rate of secretion of CMs into the circulation.6, 7 This review examines recent advances in our understanding of the regulation of CM secretion in relation to mobilization of intestinal lipid stores, with a focus on post-assembly regulatory mechanisms, including enterocyte intracellular triglyceride (TG) trafficking, CM movement in the lamina propria, and CM transport by intestinal lymphatics. Our focus is on TGs and CM particles rather than other lipid moieties.
Brief Overview of Dietary Fat Absorption, Chylomicron Biosynthesis, and Secretion
Dietary TG digestion starts with TG hydrolysis by lingual lipase in the mouth. In the stomach, gastric lipase and lingual lipase both contribute to TG hydrolysis, especially in the digestion of milk fat in newborns.8, 9 Pancreatic lipase is the major enzyme to catalyze TG hydrolysis in the small intestine. The digestion products monoglycerides (MGs) and fatty acids (FAs) form micelles with bile salts, which facilitates their absorption at the brush border across the apical membrane of enterocytes. Absorption of MGs and FAs is achieved through both passive diffusion and active transport. Facilitated diffusion is predominant at low concentrations, while simple diffusion is predominant at high concentrations for linoleic acid absorption by rat jejunum.10 Various transport proteins facilitate the transport process, including but not limited to CD36 and lipid binding proteins.11, 12 Intestinal lipid binding proteins are involved not only in luminal absorption of long-chain FAs (LCFAs), but also in modulating intracellular trafficking of FAs, TG resynthesis, and CM formation.12 CD36 may not play a quantitatively significant role in FA uptake, but it may induce key proteins in CM formation, thereby playing an important regulatory role in CM secretion.13 In a mouse model of diet-induced metabolic syndrome, down-regulation of CD36 by lipids was abolished and lipid sensing by CD36 was impaired, resulting in delayed induction of microsomal triglyceride transfer protein (MTP), liver-type-fatty acid binding protein (FABP), and apolipoprotein C-II, and aberrant TG-rich lipoprotein formation.14 CD36-/- mice have decreased lymph flow,15, 16 suggesting CD36 involvement in post-assembly transport of CMs. Polymorphism in FABP2 (encoding intestinal FABP) is associated with exaggerated postprandial plasma and CM TG response in human beings.17 Mice deficient in intestinal Fabp affects weight gain in a sex-dependent manner,18 which may underlie sexual dimorphism in lipid and lipoprotein metabolism, although gender differences in CM synthesis and secretion has not been studied extensively.
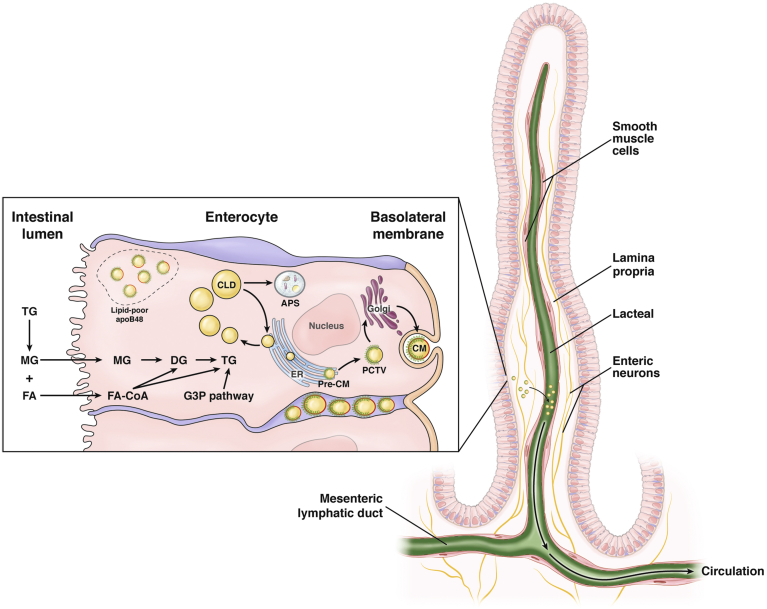
Upon entry into enterocytes, MGs and FAs are re-esterified to form TGs in the endoplasmic reticulum (ER) membrane leaflet. TG resynthesis occurs primarily (approximately 80%) through the monoacylglycerol pathway. MGs first combine with a FA to form diglycerides catalyzed by acyl CoA:monoacylglycerol acyltransferase, followed by TG formation, with the addition of a second FA catalyzed by acyl-CoA:diacylglycerol acyltransferase. The glycerol-3-phosphate pathway using glycerol-phosphate acyltransferase contributes approximately 20% to intestinal TG synthesis.19 TGs synthesized in the ER membrane form lipid droplets that either bud off to form cytoplasmic lipid droplets (CLDs) encased in a phospholipid monolayer, or secreted into the ER lumen for CM synthesis. Formation of pre-CM occurs at the inner ER membrane where apolipoprotein (apo) B48 is lipidated, facilitated by MTP. The prevailing model of CM lipidation depicts the formation of a poorly lipidated, dense, apoB48-containing particle and an apoB48-free lipid droplet, with subsequent fusion of the 2 to form the pre-CM. ApoAIV also is added to pre-CM in the ER and plays an important role in CM size and metabolism in the circulation.20 Pre-CMs are transported in special transport vesicles (pre-CM transport vesicle [PCTV]) from the ER to the Golgi apparatus, where they are processed further into mature CMs. Mature CMs exit enterocytes at the basolateral membrane, enter the lamina propria and then the lacteals, and move through the mesenteric lymphatic ducts to the thoracic duct where they enter the venous circulation at the left subclavian vein.
The following discussion focuses on TG trafficking, storage, and mobilization in the small intestine, with an emphasis on nodes of regulation in CM secretion postassembly of nascent lipoproteins (Figure 1).
Figure 1.
Triglyceride trafficking, storage, and mobilization in the small intestine. TGs are present in various pools in intracellular and extracellular spaces within the intestinal structure. Digestion products of dietary TGs are absorbed at the brush border across the apical membrane of the enterocyte. TG resynthesis through the MGAT (majority) or the G3P pathways occurs in the outer ER membrane. Lipid droplets formed in the ER membrane are packaged into pre-CMs that are transported in PCTVs to the Golgi for further processing. Mature CM particles exit the basolateral membrane via exocytosis of secretory vesicles. Lipid droplets in the ER membrane also may bud off to form CLDs that are recruited for CM synthesis during the interprandial period or are degraded in the autophagosome (APS). A putative, apical/subapical pool of lipid-poor apoB48 may be recruited for rapid CM secretion and replenished by lipid supply during the feeding–fasting cycle. Secreted CM particles move through the lamina propria, enter the lacteals, and are actively transported in lymphatic vessels of increasing size before being released into circulation. CM entry into lacteals occurs mainly through size exclusion, but certain prerequisites such as particular CM composition also play a role. SMC around the lacteal and larger lymphatic vessels, which may be modulated by neural inputs, VEGF-C, and other regulators, actively regulates lymph flow and CM transport to the circulation. CoA, coenzyme A; DG, diacylglycerol.
Transport of Pre-CMs From ER to Golgi
Pre-CMs are packaged into PCTVs, which bud off the ER membrane and move to the cis-Golgi. Pre-CM exiting the ER is considered the rate-limiting step for intracellular trafficking of TGs and is a multistep process.6 FABP1 binds to the ER to initiate PCTV formation, and together with CD36, VAMP7, and apoB48, facilitates PCTV budding. Transport of pre-CMs in PCTVs requires the soluble NSF attachment protein receptor (SNARE) protein complex. Transport vesicles use their vesicle SNARE (vSNARE) to direct the vesicles to their target, where vSNAREs pair with the target SNARE of the target membrane to form a SNARE complex to deliver the vesicle contents to target lumen via membrane fusion. The SNARE complex is composed of 4 helices, 1 from the transport vesicle and 3 from the target membrane. After budding from the ER, PCTVs are directed by vSNARE toward the Golgi. VAMP7 of vSNARE joins with syntaxin-5, rbet1, and vti1a of target SNARE to form the SNARE complex, which facilitates docking and fusion of PCTV with the Golgi membrane to release CM cargo into the Golgi lumen.21 The regulation of this machinery has not been fully defined. Our recent study suggests that syntaxin-binding protein 5 may be involved in oral glucose mobilization of intestinal lipid stores.22 A plausible mechanism is that binding of syntaxin-binding protein 5 closes syntaxin-5 and prevents the formation of the SNARE complex, PCTV fusion with the Golgi membrane, and uptake into the Golgi.
Other proteins (eg, apoAI) are transported by cytoplasmic coat protein complex II vesicles from the ER to the Golgi. cytoplasmic coat protein complex II is not needed for pre-CM to exit the ER but is needed for Golgi fusion of PCTVs. In the Golgi, maturation of CMs occurs with acquisition of additional apolipoproteins (eg, apoAI and apoAIV), and apoB48 glycosylation. Mature CMs exit the Golgi into the cytosol in a large vesicle by an unknown mechanism, before being exocytosed at the basolateral membrane. The basement membrane beneath the enterocytes may become leaky during active lipid absorption to facilitate the movement of CMs from the intercellular space to the lamina propria.23
Despite many similarities in the synthesis of CMs in enterocytes and very-low-density lipoprotein (VLDL) in hepatocytes, their intracellular transport differs in several aspects. VLDL synthesis in hepatocytes starts with MTP-dependent lipidation of nascent apoB100 to generate lipid-poor, primordial VLDL particles in the ER lumen. Lipidation of apoB100 depends on the availability of TGs, which may be derived from several sources, including free fatty acids from adipose hydrolysis, CM remnant uptake, and de novo lipogenesis. Further lipid enrichment and maturation may occur via fusion with preformed lipid droplets in the ER and in the Golgi before secretion.24, 25 ApoB100 degradation is a major regulatory mechanism, thus lipidation is necessary for stabilization of apoB100 and a reduction in fatty acid supply promotes apoB100 degradation.25, 26 Whether apoB48 protein degradation plays a major regulatory role in CM secretion is unresolved. Abundant apoB48 was observed in Caco-2 cells even without fatty acid supply, implying the lack of significant apoB48 degradation.27 On the other hand, apoB48 degradation was reported in Caco-2 cells28 and in primary hamster enterocytes.29 It is known that intestinal lipoprotein secretion occurs in rats and hamsters even in the fasted state, where a more dense, high-density lipoprotein-like or VLDL-like particle is secreted.29, 30 Transport of VLDL from the ER to the Golgi uses a distinct transport vesicle (VLDL transport vesicle), which differs from PCTV in biogenesis, size, and SNARE components in vesicle–Golgi membrane fusion.24
Intestinal Retention and Release of Lipids
A relatively recent development in the field is the recognition of postprandial lipid retention in the intestine that extends well beyond the prandial and postprandial periods. Plasma TG concentration in a normolipidemic individual after a high-fat meal usually peaks after 3–5 hours and returns to baseline by approximately 6–8 hours. Studies in human beings documented the early appearance of CM TGs after a fat-rich meal, the origin of which was attributed to lipids derived from a previous meal.31, 32, 33, 34 This time frame is earlier than that required for dietary fat digestion and absorption and CM biogenesis to occur.35 Studies with stable isotope labeling of dietary fat showed that 10%–12% of TGs from the previous meal appear in new CM within the first 20 minutes of a second meal ingestion, and that lipids from earlier meals can contribute to CM TG secretion more than 18 hours after the last meal.36 Postprandial CM TG concentrations are higher than those after an earlier meal, even when the 2 sequential meals contain exactly the same macronutrient composition, supporting a contribution of earlier dietary lipids to the appearance of CM TGs after the second meal.37 In addition, abundant lipid droplets were visible in jejunal enterocyte cytoplasm 6 hours after a high-fat liquid meal in 1 study,38 and we recently showed CLDs present in duodenal enterocyte cytoplasm as long as 10 hours after a high-fat meal.22 In mice, enterocyte cytoplasm contains lipid depots up to 12 hours after a high-fat meal.39 These observations support the existence of an enteral source of lipid stores in the postprandial period that is derived from previous dietary fat intake. This stored enteral lipid is released in response to a second meal and other stimulatory cues (discussed in greater detail later), contributing to circulating CM TGs. Although the exact source of these intestinal lipid stores is unknown, candidate pools include lipid droplets in intracellular spaces (eg, cytoplasm, organelles, and secretory pathways), and CMs in extracellular spaces (eg, lamina propria and lacteals of the mesenteric lymphatic system).
In addition to the retention of TGs and their subsequent release in response to various stimuli, we have shown that preformed apoB48 is rapidly released from the intestine in response to the gut peptide glucagon-like peptide-2 (GLP-2).40 The apoB48 released into the circulation is predominantly in the form of CMs, but whether it is derived from a pool of unlipidated/poorly lipidated apoB48 or fully preformed CMs is not fully known. Apical distribution of apoB has been reported in rat jejunal enterocytes and Caco-2 cells.41 A small amount of apoB is reported to be released from the apical side of pig intestinal explants, which was abolished by a fat meal.42 In differentiated Caco-2 cells, apoB showed apical distribution, located within the brush-border microvilli and in the subapical region.28 This apical pool of apoB was derived from the trans-Golgi network, with lipid supply driving the export of apoB from the ER and post-Golgi targeting to the apex. Apical supply of lipid micelles rapidly depleted the apical pool of apoB, mobilizing it toward the basolateral area, whereas a continuous supply of lipids replenished it. It is important to note that, in this model, the supply of exogenous lipids did not significantly increase apoB synthesis; therefore, apoB degradation served as part of the regulation and mobilization of the pre-existing ready-to-use store of apical pool of apoB, which constitutes an additional contribution to the increased apoB secretion in this condition. Synthesis of TGs and lipid droplets, from the lipid supply on the apical side, in the ER in the subapical compartment triggers the trafficking of apical apoB toward the basolateral side. The apical pool of apoB may be in the form of primordial lipoproteins, ready to be greatly expanded in size within the secretory pathway after an influx of dietary lipids. This repeated replenishment and recruitment of an apical pool of apoB48 during the feeding-fasting cycle is very interesting. The presence of an apical pool of apoB (ie, polarized distribution) is believed to be cell-specific and has not been described for hepatocytes. It remains to be established whether this process is altered in different metabolic conditions, such as the metabolic syndrome and type 2 diabetes. It is not known whether the basolateral supply of lipids also promotes the mobilization of the apical apoB pool. It also remains to be examined whether other stimuli (eg, glucose, both an apical and basolateral supply; a cephalic phase response; or GLP-2 in vivo) mobilizes this apical pool of apoB in a similar fashion. As we have shown previously, GLP-2 promotes the rapid release of preformed CM particles in which apoB48 was not labeled with stable isotope.40 It is intriguing to speculate that the apical pool of apoB48 was mobilized by GLP-2.
CLD Metabolism
Lipid droplets that are formed in the ER membrane undergo 1 of 2 fates: CM synthesis in the ER lumen or formation of CLDs. CLDs consist of a neutral lipid core containing mostly TGs and some cholesteryl ester, surrounded by a phospholipid monolayer and associated proteins. CLDs undergo dynamic synthesis, metabolism, and catabolism.43 Newly synthesized TGs accumulate within the ER membrane and promote the budding of a CLD, with CLDs further expanding in size via either fusion or TG synthesis at the CLD surface. Several enzymes that facilitate CLD synthesis are associated with the CLD membrane.43 The catabolism of CLDs within enterocytes can occur either through cytoplasmic TG lipolysis or lipophagy. CLD surface proteins (eg, ATGL, which catalyzes the hydrolysis of TG, yielding diglycerides and FAs) and its cofactor comparative gene identification-58 play important roles in cytoplasmic TG hydrolysis in enterocytes.44, 45 Autophagy plays an important role in TG targeting to lysosomes, regulating TG distribution, trafficking, and turnover in enterocytes.46 Numerous CLD-membrane–associated proteins have been identified using proteomics in Caco-2 cells after incubation with lipid micelles.47, 48 Many of these proteins are involved in lipid and lipoprotein metabolism pathways, for example, fatty acid activation, TG hydrolysis, phosphatidylcholine synthesis, steroid metabolism, and lipoprotein assembly.49 Similar findings have been reported for the proteome of CLDs isolated from mouse enterocytes harvested after a dietary fat challenge.50 Multiple physiological roles have been proposed for CLDs, including serving as transient storage of TGs in enterocytes, attenuating postprandial TG excursions, and ensuring a continuous supply of TGs for postprandial CM secretion.49 In mouse enterocytes, TG storage in CLDs is highly dynamic during dietary fat absorption, accumulating postprandially followed by gradual depletion.39 Dynamic metabolism of CLDs is believed to enhance the overall efficiency of dietary fat absorption and prevent lipid overload–induced toxicity within enterocytes and systemically.7
Emerging evidence is beginning to show the intricacies of the regulation of CLD storage and mobilization in enterocytes. Many CLD-associated proteins may play regulatory roles on CLD metabolism.43 Several members of the perilipin (Plin) family are associated with enterocyte CLDs. Plin3 localizes to CLDs after an acute dietary fat challenge, while Plin2 was found to associate only with enterocyte CLDs after chronic high-fat feeding.51 Plin2-deficient mice have decreased TG storage in enterocyte CLDs compared with wild-type mice,52 supporting a role of Plin2 in stabilizing CLDs. Cell death-inducing DFFA-like effector b (a member of a protein family with known roles in CLD fusion that localizes to both CLD and ER) may play a role in mobilizing TGs stored within enterocyte CLDs to provide substrates for CM secretion because cell death-inducing DFFA-like effector b-deficient mice show reduced intestinal TG secretion, decreased CM size, and increased TG storage within enterocyte CLDs.53 In mice, Mfge8 on the enterocyte surface regulates CLD metabolism. In response to a fat challenge, Mfge8 interacts with αvβ3 and αvβ5 integrins to increase intracellular TG hydrolase activity, enhancing CLD TG hydrolysis and CM synthesis.54 Deficiency in lysophosphatidylcholine acyltransferase-3 (a phospholipid remodeling protein) increases intestinal TG storage and reduces intestinal TG secretion in mice.55, 56, 57 Biliary phospholipid secretion supports CM membrane formation and mucosal protein synthesis,58 and CM particle size is related inversely to biliary phospholipid secretion in mice.59 This evidence supports a role of altered membrane phospholipid composition in CLD and CM mobilization. Transmembrane 6 superfamily 2-deficiency in zebrafish enterocytes and in Caco-2 cells results in CLD accumulation in response to a lipid load,60 suggesting a role in regulating CLD metabolism and intestinal TG secretion.
The exact mechanisms whereby these proteins regulate enterocyte CLD storage require further elucidation, but there is little doubt that the dynamic formation and mobilization of CLD lipids is highly regulated and plays an important role in determining the net secretion rate of CMs from the intestine.
CMs in the Lamina Propria
The lamina propria contains numerous CMs as they journey from the enterocyte to the lacteal.61 The lamina propria is a layer of loose connective tissue situated under the basolateral membrane of enterocytes and below the basement membrane, forming the intestinal mucosa together with the epithelium. Interlacing, loosely organized fibers form the areolar, sponge-like, elastic tissue with ample open space for interstitial fluid. The lamina propria is highly vascularized with blood capillaries and lymphatics. The blood vessels provide nutrient supply, waste removal, and leukocytes. Lymphatics penetrate the mucosa and lie below the basement membrane of the epithelium, from there they drain the lamina propria. In the small intestine, a central lacteal (a single, blind-ended, lymphatic vessel) lies in the center of each villus. The lamina propria also houses a rich variety of cells (eg, fibroblasts, lymphocytes, leukocytes, mast cells, and smooth muscle cells). It has been suggested that myofibroblasts also reside in the lamina propria.62 These cells have characteristics of both smooth muscle cells and fibroblasts, release cytokines and chemokines in response to stress, and play important roles in inflammation. In addition, their contractile capacity may help draw tissue together in wound healing responses. Afferent and efferent nerve endings can be found in the lamina propria as well.
Control of CM passage through the lamina propria has been relatively unappreciated; however, it may represent an added node of regulation for CM secretion. During active absorption, CM movement through the lamina propria may be by diffusion and greatly facilitated by convective fluid movement as a result of interstitial hydration.23 Blood supply to the small intestinal mucosa is through 3 main blood vessels: the celiac, superior, and inferior mesenteric arteries. Nitric oxide (NO) signaling is an important regulator of intestinal blood flow.63 The majority of NO in the intestinal mucosa is synthesized in endothelial cells by endothelial NO synthase (eNOS) using oxygen and L-arginine as substrates. Postprandially, intestinal blood flow is increased dramatically by luminal nutrients, particularly lipids and carbohydrates. NO plays an important role in hyperemia in response to luminal glucose, thus glucose absorption across the mucosa causes artery dilation with increased venous and arterial NO concentrations in the intestine,64 which can be ablated by inhibition of NOS.65 Adenosine is a potent vasodilator in the intestine, through activation of A1 or A2B receptors and downstream induction of NO.65 In addition, endogenous availability and intracellular and extracellular concentrations of NO may be regulated by cytochrome c oxidase.66 The intestinal microvasculature is subjected to neural regulation via coordinated extrinsic and enteric innervation.67 Specifically, vasoconstriction is mediated by sympathetic nerves that originate from the celiac and mesenteric ganglia and act on submucosal arterioles by releasing adenosine triphosphate onto arteriolar purinoceptors. Release of acetylcholine and/or neuropeptides from intrinsic submucosal neurons and release of substance P and calcitonin gene–related peptide from extrinsic sensory nerves lead to neurogenic vasodilation of submucosal arterioles. These vasodilatory pathways can be activated independently by mucosal stimulation, and both have afferent and efferent components confined to the mucosa and submucosal neuronal plexus. It has been postulated that the intrinsic enteric cholinergic reflex pathways mediate local physiological control of mucosal blood flow, whereas extrinsic sensory reflex pathways are preferentially activated during inflammatory states.67 Furthermore, oxygen from the arterial blood supply diffuses to adjacent venules along the crypt villus axis, resulting in decreasing levels of oxygen along the radial axis from the intestinal submucosa to the lumen, rendering the epithelial cells under “physiologic hypoxia.”68, 69 CMs are present in the lamina propria of human jejunum hours after high-fat intake and are depleted after oral glucose ingestion.38 The quantitative contribution of this pool to the overall lipid release from the intestine is unknown, but abundant CMs are present in the lamina propria in the jejunum of both rats61, 70 and human beings after fat ingestion.38 The exact mechanism regulating CM passage through the lamina propria has not been fully elucidated. Hypothetically, potential regulators may include modulation of blood flow, vasodilation, and fiber contraction, with the possible involvement of neural signals, to empty CMs into lacteals. Changes in hypoxia status in the lamina propria and in enterocytes as a result of changes in blood flow may contribute to mobilization of local CM pools. Furthermore, oral glucose– and GLP-2–mediated CM mobilization could include NO-dependent (blood flow) and NO-independent (eg, modulation of hypoxia) mechanisms, which warrants future investigation.
Role of Regulated Enteric Lymphatic Flow in Chylomicron Secretion
The intestinal lymphatic vasculature plays an important role in body fluid homeostasis, dietary fat absorption, and gut immune responses.71 For in-depth discussion on the functions and metabolic implications of the intestinal lymphatic system, readers are referred to a comprehensive review in this issue.72 For a complete discussion on post-assembly control of CM secretion, the role of intestinal lymphatics on lipid handling is discussed briefly here.
The majority of CMs in the lamina propria are taken up into lacteals.70 It generally is believed that CMs enter lacteals through size exclusion. The paracellular pathway is likely the main route for CMs to enter lacteals, where intercellular junctions at the tip of the lacteal are large enough to allow entry of CMs of considerable sizes down their concentration gradient, although transcellular transport also has been proposed.73 A small fraction of CMs of much smaller size may enter subepithelial blood capillaries, which may involve VLDL receptors on endothelial cells.61 CMs in lacteals drain into mesenteric lymphatic ducts and are transported to the thoracic duct before being released into the circulation at the left subclavian vein.
Regulation of CM uptake and transport in the lymphatic vasculature is beginning to be elucidated. CM uptake into the lacteals may be more complex than simple size exclusion. For example, in mice lacking the transcription factor pleomorphic adenoma gene-like 2, CMs can exit enterocytes but fail to enter lacteals, and thus accumulate in the lamina propria.74 The small portion of CMs that do reach the circulation are not efficiently taken up and metabolized by local tissues, resulting in an inability to absorb fat, postnatal wasting, and death. The mechanism whereby this occurs remains unknown but may be owing to generation of CMs with compositional properties that allow them to exit the enterocytes but fail to enter lacteals. Consistent with this hypothesis, pleomorphic adenoma gene-like 2 expression is limited to enterocytes and its deficiency is associated with reduced expression of several genes (eg, sorting nexins and vacuolar sorting proteins) that are candidate regulators of intracellular steps of CM assembly. This implies that the lacteals are able to discriminate CMs not only by particle size but also by particle composition. In addition, lacteal permeability is under molecular control, thus deletion of endothelial cell receptors neuropilin-1 and vascular endothelial growth factor receptor 1 closes lacteal junctions and prevents lacteal CM uptake.75
Recent reports have suggested that lymphatics play an active role in lipid transport and that compromised lymphatic function bears systemic consequences to lipid metabolism and transport.73 Contrary to the belief that the lymphatic vasculature is a passive conduit, the flow of lymph in the lymphatic vessel is in large part achieved through an active pumping mechanism.76, 77 The lymphatic smooth muscles show both tonic (vessel diameter) and phasic (frequency and amplitude) contractions, which, together with the 1-way valves that prevent backflow, actively transport lymph from the intestine to the circulation. Lymphatic pumping function may be affected by lipid load, thus both tonic and phasic contractility of mesenteric lymphatics are reduced with infusion of lipids into the duodenum of rats, although total lymph flow is increased.76 Active contraction of lacteals also participates in dietary lipid drainage in mice.78 Contraction of smooth muscles that surround each lacteal is under the control of the autonomic nervous system.78 Importantly, lymphatic function is impaired in the metabolic syndrome, thus lymphatics from rats with the metabolic syndrome have reduced potential load capabilities and impaired intrinsic contractility required for proper lymph flow.79, 80 Collectively, this evidence supports an active role of intestinal lymphatics, including lacteals and mesenteric lymphatic ducts, in physiological control of CM secretion. This node of regulation adds an additional layer of complexity to our attempts to elucidate the mechanisms of CM secretion. Understanding the regulation of lymphatic flow and its contribution to intestinal lipid absorption and CM secretion is still in its infancy.
Molecular Regulation of Intestinal Lymphatic Function
The development and maintenance of intestinal lymphatic vasculature are under molecular regulation. Vascular endothelial growth factor-C (VEGF-C), through activation of VEGF receptor 3 (VEGFR3), is a key regulator of lymphatic vessel growth during development and pathologic processes.81 The maintenance of the intestinal lymphatic architecture requires VEGF-C whereas Vegfc deletion leads to atrophy of the intestinal lymphatic vasculature, including lacteals, and defective lipid absorption.82 In the intestine, VEGF-C is expressed by a subset of smooth muscle cells (SMCs) adjacent to the lacteals in the villus and in the intestinal wall, and activated by proteolytic cleavage of the full-length protein.83 VEGF-C is necessary for perinatal lymphangiogenesis, including in the small intestine,84 however, in adult mice it is required for lymphatic vessel maintenance only in the intestine.82 The adult intestinal lacteals, unlike other lymphatics, are in a permanent regenerative and proliferative state, undergoing continuous remodeling. Regeneration and maintenance of lacteals is mediated by Notch signaling, involving Notch ligand delta-like 4 whose expression requires activation of VEGFR3 and VEGFR2.85 VEGF-C is known to stimulate lymphatic pump activation by inducing contraction of SMCs surrounding the collecting lymphatic vessels,86 and SMC contractility in the villi is important for dietary lipid absorption.87 Calcitonin receptor-like receptor (CLR) is a component of the adrenomedullin signaling pathway that is important for lymphangiogenesis during development.88 In adult mice, genetic ablation of lymphatic Calcrl (encoding CLR) induces systemic lymphatic insufficiency, intestinal inflammation, and intestinal lymphatic dilation. Importantly, these mice show reduced proliferation of lymphatic endothelial lacteals and accumulation of lipids toward the center of the villi or within the submucosal area, suggesting an essential role of CLR expression in lipid absorption within the intestinal lacteals.89 Calcrl deletion also down-regulates the expression of the Notch ligand delta-like protein 4 (activation of which by VEGFR2 and VEGFR3 is important for the regeneration and functions of lacteals). These data support that a Calcrl-Notch-delta-like protein 4 signaling cascade is important for the maintenance of intestinal lymphatic function. Collectively, molecular control of intestinal lymphatic architecture, inflammation, and function in adults is highly regulated. Disruption of molecular pathways that regulate the development and maintenance of the lymphatic vasculature and intestinal inflammation compromises lipid absorption into lacteals and lipid handling in the gut in general.
Known Regulators of Intestinal TG Mobilization
Cephalic Phase Release of CMs: Sequential Meal and Sham Fat Feeding
It generally is recognized that a cephalic phase of gastric secretion exists, before food reaching the stomach, usually triggered by seeing, thinking, and tasting of food. Cephalic responses have been described for the secretion of hormones (eg, insulin,90 ghrelin, and pancreatic polypeptide).91, 92, 93 An early peak (within in an hour) in plasma and CM TGs was observed with the ingestion of a high-fat meal that follows an earlier high-fat meal, which reflects a fatty acid profile of the earlier meal.31, 33 This suggests that a pool of lipids originating from the previous meal is recruited for secretion in CM particles after the intake of the second meal. This CM secretion occurs before absorption of dietary lipids in the second meal, implying mobilization of a pre-existing pool. The site of this lipid store is as yet unidentified, with consideration given to both intracellular and extracellular lipid pools.
The cephalic phase of CM secretion has been described with oral fat tasting (without swallowing). In human beings, oral exposure to nutrients, especially fat, increases serum TGs.94 Sham fat feeding prompted the appearance of several sequential plasma TG peaks, the earliest within 30 minutes of oral fat tasting.37 The signal to initiate this rapid, cephalic response has not been fully defined. Cephalic phase secretion of hormones (insulin, ghrelin, and pancreatic polypeptide secretion) may involve the vagal nerve.91 A cephalic phase response of insulin secretion may involve both cholinergic and noncholinergic autonomic activation.90 Sham fat feeding is associated with cephalic phase responses, including release of pancreatic polypeptide, insulin, and CM TGs, which is believed to be related to vagal stimulation and increased parasympathetic activity.93 Such a mechanism has not been examined for the cephalic phase of CM secretion. The initiation of this process may start with oral taste receptors sensing fatty acids.95 There is evidence to suggest the involvement of CD36. CD36 is expressed on the apical side of taste bud cells in the lingual epithelium and enhances bile secretion in response to tasting unsaturated LCFAs.96 Orosensory perception of LCFAs in mouse requires CD36.97 It has been proposed that binding of LCFA to CD36 on taste bud cells initiates signaling to the afferent nerve fibers and transmits the output signal from taste buds to the central nervous system.98 Common variants in the CD36 gene are associated with fat perception and preference, such as those prevalent in African Americans.99 It would be interesting to see whether such variants are associated with a different cephalic phase of intestinal lipid release and CM secretion.
Oral Glucose Is a Known Stimulus of Lipid and CM Release From the Intestine
Oral glucose has been shown to mobilize intestinal lipid stores, but the underlying cellular and molecular mechanisms remain unclear. In human jejunal biopsy specimens, large quantities of lipid droplets are visible in enterocytes and lamina propria 6 hours after ingestion of a high-fat liquid meal.38 When a glucose solution was ingested 5 hours after the high-fat meal and 1 hour before the biopsy, lipid droplets were largely cleared from the enterocytes. This was accompanied by increases in plasma TGs and CM TGs. In a recent study, we re-examined this phenomenon.22 Healthy volunteers ingested a high-fat liquid meal, followed 5 hours later by ingestion of water or glucose. Plasma and CM TGs were measured for 3 hours after glucose or water ingestion. Our study confirmed the existence of plasma and CM TG peaks after glucose ingestion. In a separate cohort, duodenal biopsy specimens were obtained 1 hour after glucose or water ingestion (ie, 6 hours after high-fat formula ingestion). With water ingestion, considerable quantities of lipids were present in enterocytes in the form of CLDs. Glucose ingestion caused quantitative and qualitative changes in CLDs, with fewer CLDs present and a shift in CLD size toward smaller CLDs compared with water ingestion. The effects of glucose ingestion are dependent on sufficient lipid stores within the enterocyte because oral glucose did not reduce intestinal CLD stores after a 10-hour fast, at which time CLD stores still were present in enterocytes but largely diminished compared with 6 hours after fat ingestion. These results show that a considerable amount of dietary lipid is retained in intestinal CLDs well into the late postprandial period and that mobilization of intestinal lipid stores by oral glucose occurs in part by mobilization of CLDs for CM secretion.
We further examined the proteome of the duodenal biopsy specimens. No significant changes were detected in proteins involved in CLD metabolism or lipoprotein assembly, likely because of the small sample size. However, several proteins were expressed differentially with glucose vs water ingestion. Glucose ingestion relatively up-regulated ethanolaminephosphotransferase 1 and epimerase family protein short-chain dehydrogenase/reductase family 39U member 1 (SDR39U1), and down-regulated syntaxin-binding protein 5 (STXBP5). Ethanolaminephosphotransferase 1 is involved in the synthesis of phosphatidylethanolamine.100 Mutations in several enzymes involved in phospholipid synthesis are associated with diseases including fatty liver, lipodystrophy, and obesity.101 In addition, phospholipid remodeling protein lysophosphatidylcholine acyltransferase-3 deficiency alters the phospholipid composition of CLDs, CMs, and the ER, and impacts lipid storage and secretion.55, 56, 57 Therefore, it is possible that higher levels of ethanolaminephosphotransferase 1 in the intestine in response to glucose has an impact on membrane composition of the ER and/or CMs, which ultimately promotes CM secretion. SDR39U1 is expressed in small intestine.102 It is a member of the family of enzymes participating in the metabolism of steroid hormones, prostaglandins, retinoids, lipids, and xenobiotics.103 Genetic defects in SDR genes underlie several inherited metabolic diseases.104 Syntaxin 5 involved in pre-CM trafficking and docking to the Golgi as part of the target SNARE complex at the Golgi membrane.6, 21 It is possible that, by binding to syntaxin 5, STXBP5 functions as a regulator of pre-CM docking to Golgi, regulating subsequent CM maturation and exiting the enterocyte. Glucose suppression of STXBP5 could hypothetically enhance the action of syntaxin 5, thereby enhancing the binding of PCTVs to the Golgi membrane, leading to enhanced CM secretion. The role played by these candidate molecular players in lipid mobilization and CM secretion requires further validation.
Other indirect mechanisms also may modulate glucose-mediated mobilization of intestinal lipids. In rodent models, luminal glucose increases sympathetic activity leading to vasodilation of the submucosal arterioles via adenosine A1 receptors and increases NO.65 Luminal glucose also increases fluid absorption and lymph flow in rats.105 This might be related to increased villus and submucosal lymph osmolarity and flow with luminal glucose supply.106 In addition, ingestion of a carbohydrate-rich meal increased sympathetic nerve activity and vasodilation in human beings.107 Finally, glucose ingestion may stimulate GLP-2 secretion, which per se has stimulatory effects on CM release (as discussed later). In healthy subjects undergoing a standard OGTT, the plasma GLP-2 concentration increased from approximately 15 to 49 pmol/L.108 The plasma GLP-2 concentration increased to approximately 50 pmol/L after a mixed meal and to nearly 1500 pmol/L after subcutaneous injection of 400 µg GLP-2.109 The amount of glucose ingested in the earlier-described study (25 g) was much smaller than a standard OGTT (75 g), and GLP-2 stimulation of CM release was observed with subcutaneous injection of 1500 µg GLP-2.40 These data suggest a less likely role of GLP-2 in mediating the effects of oral glucose on mobilizing intestinal lipid stores. The effects of oral glucose on gut lipid mobilization in relation to modulation of lymphatic function has not been examined in depth in human beings.
The Gut Hormone GLP-2 Is a Known Potent Stimulus of Intestinal Lipid Mobilization and CM Secretion
GLP-2 acutely enhances CM secretion in hamsters and mice.110 This was shown to be owing in part to accelerated dietary fatty acid uptake via increased CD36/FA translocase glycosylation in the enterocyte. GLP-2 also increased circulating CMs in hamsters when administered 5 hours after infusion of oil into the duodenum, suggesting mobilization of intestinal lipid stores. In human beings, GLP-2 infusion potentiated postprandial TG excursion and increased circulating FAs.111 In healthy human beings, we showed that a single subcutaneous injection of GLP-2 resulted in rapid and transient increases in plasma and CM TGs and apoB48 under conditions of constant intraduodenal lipid infusion and a pancreatic clamp, the latter preventing acute excursions in insulin, glucagon, and growth hormone.40 This effect also was observed when administered 7 hours after a high-fat meal containing retinyl palmitate as a label of dietary fats. The increase in retinyl palmitate in parallel to TGs in plasma and CM indicated that GLP-2 promoted the release of TGs in preformed CMs that originated from the earlier fat-containing meal. These results show a novel mechanism whereby GLP-2 signaling gains access to intestinal lipid store pool(s).
The mechanism whereby GLP-2 mobilizes CM release remains unknown. Several lines of evidence suggest that GLP-2 may target postenterocyte lipid pools. First, although still being debated, it has become evident that the GLP-2 receptor (GLP-2R) is not expressed in enterocytes where CM synthesis occurs.112, 113 GLP-2R is expressed prominently in the gastrointestinal tract, but extraintestinal expression including in the central nervous system also has been documented.114, 115, 116 Recently, the exact distribution of intestinal GLP-2R was mapped in the mouse.117 Within the duodenum, GLP-2R was expressed abundantly in the lamina propria of the mucosa layer and in the circular and longitudinal muscle layers. Glp2r messenger RNA (mRNA) transcripts were localized to the nerve plexuses in the caudal duodenum. In the jejunum and ileum, GLP-2R was found to be highly expressed in scattered cells within the mucosa and the nerve plexus, with sporadic expression in the muscle cells of the muscularis layer. In the cecum and colon, Glp2r mRNA was expressed in both the mucosa and nerve plexus. In addition to the expression observed in the myenteric plexuses, GLP-2R also was detected in submucosal plexuses of the colon. Throughout the intestinal tract, Glp2r mRNA was expressed markedly in the mucosal lamina propria, consistent with the distribution of subepithelial myofibroblasts.112, 118 These expression patterns suggest that GLP-2 primarily targets the intestinal structure below the enterocytes, with potential roles in modulating muscular contractility and enteric nervous system (ENS) activity.
The ENS may be involved in GLP-2–stimulated CM secretion. The ENS interacts with the gut endocrine system and projects into the intestinal mucosa where enterocytes are located.119 Several cell types in the intestine have been identified with GLP-2R expression, including subepithelial myofibroblasts,118 enteroendocrine cells,114 and enteric neurons that express eNOS and are positive for vasoactive intestinal polypeptide.115 These cells are richly present in the submucosal regions, including the lamina propria. GLP-2 has been shown to activate enteric neurons in both rats and mice and modulate duodenal muscle contractility by increasing NO production and decreasing cholinergic neurotransmission.120, 121 Furthermore, GLP-2 increases the expression of neuronal NOS and vasoactive intestinal polypeptide in enteric neurons,122 2 regulators of intestinal vasodilation and blood flow that may indirectly influence lipid transport through the surrounding intestinal regions. This supports a role of GLP-2 signaling through receptor expression in the ENS and the subepithelial mucosa to modulate CM secretion, and this process may involve GLP-2 modulation of the intestinal vasculature including the blood vessels and the lymphatics in the gut. A plausible model would involve GLP-2 action via paracrine or endocrine mechanisms to stimulate mucosal mobility and vasodilation for the release of the CM pool in the lamina propria and lacteals.
GLP-2 is a potent regulator of intestinal blood flow. GLP-2 increases mesenteric blood flow in healthy human beings and in patients with short-bowel syndrome,123, 124 in piglets,125 and in rats.126 Several studies in animals have suggested that GLP-2 modulation of CM secretion is NO-dependent. For instance, GLP-2 increased jejunal NO metabolite levels in hamsters, suggesting the activation of NOS.127 Inhibition of NOS reduces TG-rich lipoprotein TG levels, while supply of physiological NO donor increased apoB48 secretion, suggesting that NO directly enhances enterocyte CM secretion.127 GLP-2–stimulated release of stored TGs from the enterocyte is suggested to be at least in part through NO, and this process may partly rely on MTP activity.127 It also is important to point out that GLP-2 increased not only CM TGs but also apoB48 in human beings,40 pointing to effects on mobilization of CMs. In our recent study in human beings, the NOS inhibitor NG-monomethyl-l-arginine blocked GLP-2–mediated stimulation of intestinal arterial blood flow without significant attenuation of CM secretion,128 raising the question of the translatability of the findings from animal models to human beings. There are some caveats in drawing this conclusion. In the human study, NO production, especially that in the localized subepithelial regions such as areas in the lamina propria surrounding the lacteals, cannot be assessed and may not have been fully blocked by a NOS inhibitor. It is possible that GLP-2 regulation of CM secretion is confined in a locally NO-dependent, blood flow–independent manner. Furthermore, NO production has multiple pathways involving 1 of 3 NOS isoforms in the mammalian system, and the specificity of NG-monomethyl-l-arginine on each of these cannot be assessed in vivo. Finally, GLP-2 also may stimulate intestinal subepithelial myofibroblast cells to release VEGF-C,129 and VEGFR2 signaling can activate eNOS.130 Considering the role of VEGF-C in regulating lymphatic functions, GLP-2 may modulate lymphatic vasculature in the intestinal region, which has not yet been established. Future studies are required to examine the potential involvement of the mesenteric lymphatic vasculature, including the lacteals, in GLP-2 stimulation in post-enterocyte CM mobilization.
One additional potential mechanism of GLP-2 stimulation of intestinal lipid mobilization and CM secretion bears consideration. Glp2r mRNA and protein are highly expressed in the arcuate nucleus and dorsomedial nucleus of the mouse hypothalamus.116 Central GLP-2 acts on pro-opiomelanocortin neurons to suppress food intake, gastric motility, and hepatic glucose production.116, 131, 132 The central nervous system provides extrinsic neural inputs that regulate, modulate, and control gastrointestinal functions,133 and central regulation of CM secretion has been proposed134; however, solid evidence is lacking to support that GLP-2 modulates CM mobilization through a central pathway.
Conclusions and Future Directions
Fat ingestion is overwhelmingly the major regulator of CM secretion but a second tier of fine regulation by hormones, nutrients, neural stimuli, and pharmacologic agents increasingly is being recognized, and dysregulation of these mechanisms in conditions such as insulin-resistant states is becoming evident.2, 3 There is increasing recognition that, besides the already well-described nodes of regulation of enterocyte CM assembly and secretion, post-assembly nodes of regulation also may play important roles in CM secretion. Although research in these areas is starting to show regulatory mechanisms related to each step, our understanding is still rudimentary and many questions remain to be answered. For example, what is the quantitative contribution or size of the intestinal TG store that can be mobilized? Quantitatively, which lipid pool is mobilized by the various stimuli discussed in this review and are the various pools recruited simultaneously or differentially? Do different stimuli target distinct lipid store pools and are their stimulatory effects additive or synergistic? Preliminary data in rats suggest that GLP-2 effects may be quantitatively larger than those of glucose; however, there has been no head-to-head comparison between these 2 stimuli to date. It also remains to be established whether TG stores and mobilization in the intestine are indicative of metabolic status (ie, whether they are compromised or overactive in compromised metabolic conditions, eg, diabetes, metabolic syndrome, and insulin resistance). Can potential modalities be developed to safely target intestinal lipid storage and mobilization for health benefits? These are just a few of the many questions that arise from our growing understanding of the complexity of intestinal lipid mobilization and CM secretion. We hope that improved understanding of intestinal lipid storage and mobilization will provide unique opportunities that ultimately have translational value for dietary guidelines, the prevention and treatment of dyslipidemia and cardiovascular disease, and improve health in general.
Footnotes
Author contributions Changting Xiao and Gary F. Lewis wrote the manuscript; and Changting Xiao, Priska Stahel, and Gary F. Lewis revised the manuscript.
Conflicts of interest The authors disclose no conflicts.
Funding This work was supported by an operating grant from the Canadian Institutes of Health Research, the Drucker Family Chair in Diabetes Research and the Sun Life Financial Chair in Diabetes (G.F.L.), and a Diabetes Action Canada Postdoctoral Fellowship award (P.S.).
Contributor Information
Changting Xiao, Email: ctxiao@uhnresearch.utoronto.ca.
Gary F. Lewis, Email: gary.lewis@uhn.ca.
References
- 1.Abumrad N.A., Davidson N.O. Role of the gut in lipid homeostasis. Physiol Rev. 2012;92:1061–1085. doi: 10.1152/physrev.00019.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewis G.F., Xiao C., Hegele R.A. Hypertriglyceridemia in the genomic era: a new paradigm. Endocr Rev. 2015;36:131–147. doi: 10.1210/er.2014-1062. [DOI] [PubMed] [Google Scholar]
- 3.Dash S., Xiao C., Morgantini C., Lewis G.F. New insights into the regulation of chylomicron production. Annu Rev Nutr. 2015;35:265–294. doi: 10.1146/annurev-nutr-071714-034338. [DOI] [PubMed] [Google Scholar]
- 4.Hussain M.M. Intestinal lipid absorption and lipoprotein formation. Curr Opin Lipidol. 2014;25:200–206. doi: 10.1097/MOL.0000000000000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mansbach C.M., Siddiqi S.A. The biogenesis of chylomicrons. Annu Rev Physiol. 2010;72:315–333. doi: 10.1146/annurev-physiol-021909-135801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mansbach C.M., Siddiqi S. Control of chylomicron export from the intestine. Am J Physiol Gastrointest Liver Physiol. 2016;310:G659–G668. doi: 10.1152/ajpgi.00228.2015. [DOI] [PubMed] [Google Scholar]
- 7.Xiao C., Stahel P., Carreiro A.L., Buhman K.K., Lewis G.F. Recent advances in triacylglycerol mobilization by the gut. Trends Endocrinol Metab. 2018;29:151–163. doi: 10.1016/j.tem.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Fink C.S., Hamosh P., Hamosh M. Fat digestion in the stomach: stability of lingual lipase in the gastric environment. Pediatr Res. 1984;18:248–254. doi: 10.1203/00006450-198403000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Hamosh M., Scanlon J.W., Ganot D., Likel M., Scanlon K.B., Hamosh P. Fat digestion in the newborn. Characterization of lipase in gastric aspirates of premature and term infants. J Clin Invest. 1981;67:838–846. doi: 10.1172/JCI110101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chow S.L., Hollander D. A dual, concentration-dependent absorption mechanism of linoleic acid by rat jejunum in vitro. J Lipid Res. 1979;20:349–356. [PubMed] [Google Scholar]
- 11.Cifarelli V., Abumrad N.A. Intestinal CD36 and other key proteins of lipid utilization: role in absorption and gut homeostasis. Compr Physiol. 2018;8:493–507. doi: 10.1002/cphy.c170026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buttet M., Traynard V., Tran T.T.T., Besnard P., Poirier H., Niot I. From fatty-acid sensing to chylomicron synthesis: role of intestinal lipid-binding proteins. Biochimie. 2014;96:37–47. doi: 10.1016/j.biochi.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Tran T.T.T., Poirier H., Clément L., Nassir F., Pelsers M.M.A.L., Petit V., Degrace P., Monnot M.-C., Glatz J.F.C., Abumrad N.A., Besnard P., Niot I. Luminal lipid regulates CD36 levels and downstream signaling to stimulate chylomicron synthesis. J Biol Chem. 2011;286:25201–25210. doi: 10.1074/jbc.M111.233551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buttet M., Poirier H., Traynard V., Gaire K., Tran T.T.T., Sundaresan S., Besnard P., Abumrad N.A., Niot I. Deregulated lipid sensing by intestinal CD36 in diet-induced hyperinsulinemic obese mouse model. PLoS One. 2016;11:e0145626. doi: 10.1371/journal.pone.0145626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drover V.A., Ajmal M., Nassir F., Davidson N.O., Nauli A.M., Sahoo D., Tso P., Abumrad N.A. CD36 deficiency impairs intestinal lipid secretion and clearance of chylomicrons from the blood. J Clin Invest. 2005;115:1290–1297. doi: 10.1172/JCI21514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nauli A.M., Nassir F., Zheng S., Yang Q., Lo C.-M., Vonlehmden S.B., Lee D., Jandacek R.J., Abumrad N.A., Tso P. CD36 is important for chylomicron formation and secretion and may mediate cholesterol uptake in the proximal intestine. Gastroenterology. 2006;131:1197–1207. doi: 10.1053/j.gastro.2006.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agren J.J., Valve R., Vidgren H., Laakso M., Uusitupa M. Postprandial lipemic response is modified by the polymorphism at codon 54 of the fatty acid-binding protein 2 gene. Arterioscler Thromb Vasc Biol. 1998;18:1606–1610. doi: 10.1161/01.atv.18.10.1606. [DOI] [PubMed] [Google Scholar]
- 18.Vassileva G., Huwyler L., Poirier K., Agellon L.B., Toth M.J. The intestinal fatty acid binding protein is not essential for dietary fat absorption in mice. FASEB J. 2000;14:2040–2046. doi: 10.1096/fj.99-0959com. [DOI] [PubMed] [Google Scholar]
- 19.Khatun I., Clark R.W., Vera N.B., Kou K., Erion D.M., Coskran T., Bobrowski W.F., Okerberg C., Goodwin B. Characterization of a novel intestinal glycerol-3-phosphate acyltransferase pathway and its role in lipid homeostasis. J Biol Chem. 2016;291:2602–2615. doi: 10.1074/jbc.M115.683359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kohan A.B., Wang F., Lo C.-M., Liu M., Tso P. ApoA-IV: current and emerging roles in intestinal lipid metabolism, glucose homeostasis, and satiety. Am J Physiol Gastrointest Liver Physiol. 2015;308:G472–G481. doi: 10.1152/ajpgi.00098.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siddiqi S.A., Siddiqi S., Mahan J., Peggs K., Gorelick F.S., Mansbach C.M. The identification of a novel endoplasmic reticulum to Golgi SNARE complex used by the prechylomicron transport vesicle. J Biol Chem. 2006;281:20974–20982. doi: 10.1074/jbc.M601401200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao C., Stahel P., Carreiro A.L., Hung Y.-H., Dash S., Bookman I., Buhman K.K., Lewis G.F. Oral glucose mobilizes triglyceride stores from the human intestine. Cell Mol Gastroenterol Hepatol. 2019;7:313–337. doi: 10.1016/j.jcmgh.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tso P., Balint J.A. Formation and transport of chylomicrons by enterocytes to the lymphatics. Am J Physiol. 1986;250:G715–G726. doi: 10.1152/ajpgi.1986.250.6.G715. [DOI] [PubMed] [Google Scholar]
- 24.Tiwari S., Siddiqi S.A. Intracellular trafficking and secretion of VLDL. Arterioscler Thromb Vasc Biol. 2012;32:1079–1086. doi: 10.1161/ATVBAHA.111.241471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fisher E.A., Ginsberg H.N. Complexity in the secretory pathway: the assembly and secretion of apolipoprotein B-containing lipoproteins. J Biol Chem. 2002;277:17377–17380. doi: 10.1074/jbc.R100068200. [DOI] [PubMed] [Google Scholar]
- 26.Davidson N.O., Shelness G.S. Apolipoprotein B: mRNA editing, lipoprotein assembly, and presecretory degradation. Annu Rev Nutr. 2000;20:169–193. doi: 10.1146/annurev.nutr.20.1.169. [DOI] [PubMed] [Google Scholar]
- 27.Liao W., Chan L. Apolipoprotein B, a paradigm for proteins regulated by intracellular degradation, does not undergo intracellular degradation in CaCo2 cells. J Biol Chem. 2000;275:3950–3956. doi: 10.1074/jbc.275.6.3950. [DOI] [PubMed] [Google Scholar]
- 28.Morel E., Demignot S., Chateau D., Chambaz J., Rousset M., Delers F. Lipid-dependent bidirectional traffic of apolipoprotein B in polarized enterocytes. Mol Biol Cell. 2004;15:132–141. doi: 10.1091/mbc.E03-04-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haidari M., Leung N., Mahbub F., Uffelman K.D., Kohen-Avramoglu R., Lewis G.F., Adeli K. Fasting and postprandial overproduction of intestinally derived lipoproteins in an animal model of insulin resistance. Evidence that chronic fructose feeding in the hamster is accompanied by enhanced intestinal de novo lipogenesis and ApoB48-containing lipoprotein overproduction. J Biol Chem. 2002;277:31646–31655. doi: 10.1074/jbc.M200544200. [DOI] [PubMed] [Google Scholar]
- 30.Ockner R.K., Hughes F.B., Isselbacher K.J. Very low density lipoproteins in intestinal lymph: origin, composition, and role in lipid transport in the fasting state. J Clin Invest. 1969;48:2079–2088. doi: 10.1172/JCI106174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Evans K., Kuusela P.J., Cruz M.L., Wilhelmova I., Fielding B.A., Frayn K.N. Rapid chylomicron appearance following sequential meals: effects of second meal composition. Br J Nutr. 1998;79:425–429. doi: 10.1079/bjn19980072. [DOI] [PubMed] [Google Scholar]
- 32.Jackson K.G., Robertson M.D., Fielding B.A., Frayn K.N., Williams C.M. Olive oil increases the number of triacylglycerol-rich chylomicron particles compared with other oils: an effect retained when a second standard meal is fed. Am J Clin Nutr. 2002;76:942–949. doi: 10.1093/ajcn/76.5.942. [DOI] [PubMed] [Google Scholar]
- 33.Fielding B.A., Callow J., Owen R.M., Samra J.S., Matthews D.R., Frayn K.N. Postprandial lipemia: the origin of an early peak studied by specific dietary fatty acid intake during sequential meals. Am J Clin Nutr. 1996;63:36–41. doi: 10.1093/ajcn/63.1.36. [DOI] [PubMed] [Google Scholar]
- 34.Silva K.D.R.R., Wright J.W., Williams C.M., Lovegrove J.A. Meal ingestion provokes entry of lipoproteins containing fat from the previous meal: possible metabolic implications. Eur J Nutr. 2005;44:377–383. doi: 10.1007/s00394-004-0538-3. [DOI] [PubMed] [Google Scholar]
- 35.Mansbach C.M., Nevin P. Intracellular movement of triacylglycerols in the intestine. J Lipid Res. 1998;39:963–968. [PubMed] [Google Scholar]
- 36.Chavez-Jauregui R.N., Mattes R.D., Parks E.J. Dynamics of fat absorption and effect of sham feeding on postprandial lipema. Gastroenterology. 2010;139:1538–1548. doi: 10.1053/j.gastro.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mattes R.D. Oral fat exposure increases the first phase triacylglycerol concentration due to release of stored lipid in humans. J Nutr. 2002;132:3656–3662. doi: 10.1093/jn/132.12.3656. [DOI] [PubMed] [Google Scholar]
- 38.Robertson M.D., Parkes M., Warren B.F., Ferguson D.J., Jackson K.G., Jewell D.P., Frayn K.N. Mobilisation of enterocyte fat stores by oral glucose in humans. Gut. 2003;52:834–839. doi: 10.1136/gut.52.6.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu J., Lee B., Buhman K.K., Cheng J.-X. A dynamic, cytoplasmic triacylglycerol pool in enterocytes revealed by ex vivo and in vivo coherent anti-Stokes Raman scattering imaging. J Lipid Res. 2009;50:1080–1089. doi: 10.1194/jlr.M800555-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dash S., Xiao C., Morgantini C., Connelly P.W., Patterson B.W., Lewis G.F. Glucagon-like peptide-2 regulates release of chylomicrons from the intestine. Gastroenterology. 2014;147:1275–1284. doi: 10.1053/j.gastro.2014.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levy E., Bendayan M. Use of immunoelectron microscopy and intestinal models to explore the elaboration of apolipoproteins required for intraenterocyte lipid transport. Microsc Res Tech. 2000;49:374–382. doi: 10.1002/(SICI)1097-0029(20000515)49:4<374::AID-JEMT6>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 42.Danielsen E.M., Hansen G.H., Poulsen M.D. Apical secretion of apolipoproteins from enterocytes. J Cell Biol. 1993;120:1347–1356. doi: 10.1083/jcb.120.6.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.D’Aquila T., Hung Y.-H., Carreiro A., Buhman K.K. Recent discoveries on absorption of dietary fat: presence, synthesis, and metabolism of cytoplasmic lipid droplets within enterocytes. Biochim Biophys Acta. 2016;1861:730–747. doi: 10.1016/j.bbalip.2016.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Obrowsky S., Chandak P.G., Patankar J.V., Povoden S., Schlager S., Kershaw E.E., Bogner-Strauss J.G., Hoefler G., Levak-Frank S., Kratky D. Adipose triglyceride lipase is a TG hydrolase of the small intestine and regulates intestinal PPARα signaling. J Lipid Res. 2013;54:425–435. doi: 10.1194/jlr.M031716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xie P., Guo F., Ma Y., Zhu H., Wang F., Xue B., Shi H., Yang J., Yu L. Intestinal Cgi-58 deficiency reduces postprandial lipid absorption. PLoS One. 2014;9:e91652. doi: 10.1371/journal.pone.0091652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khaldoun S.A., Emond-Boisjoly M.-A., Chateau D., Carrière V., Lacasa M., Rousset M., Demignot S., Morel E. Autophagosomes contribute to intracellular lipid distribution in enterocytes. Mol Biol Cell. 2014;25:118–132. doi: 10.1091/mbc.E13-06-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beilstein F., Bouchoux J., Rousset M., Demignot S. Proteomic analysis of lipid droplets from Caco-2/TC7 enterocytes identifies novel modulators of lipid secretion. PLoS One. 2013;8:e53017. doi: 10.1371/journal.pone.0053017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bouchoux J., Beilstein F., Pauquai T., Guerrera I.C., Chateau D., Ly N., Alqub M., Klein C., Chambaz J., Rousset M., Lacorte J.M., Morel E., Demignot S. The proteome of cytosolic lipid droplets isolated from differentiated Caco-2/TC7 enterocytes reveals cell-specific characteristics. Biol Cell. 2011;103:499–517. doi: 10.1042/BC20110024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beilstein F., Carriere V., Leturque A., Demignot S. Characteristics and functions of lipid droplets and associated proteins in enterocytes. Exp Cell Res. 2015;340:172–179. doi: 10.1016/j.yexcr.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 50.D’Aquila T., Sirohi D., Grabowski J.M., Hedrick V.E., Paul L.N., Greenberg A.S., Kuhn R.J., Buhman K.K. Characterization of the proteome of cytoplasmic lipid droplets in mouse enterocytes after a dietary fat challenge. PLoS One. 2015;10:e0126823. doi: 10.1371/journal.pone.0126823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee B., Zhu J., Wolins N.E., Cheng J.-X., Buhman K.K. Differential association of adipophilin and TIP47 proteins with cytoplasmic lipid droplets in mouse enterocytes during dietary fat absorption. Biochim Biophys Acta. 2009;1791:1173–1180. doi: 10.1016/j.bbalip.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 52.Frank D.N., Bales E.S., Monks J., Jackman M.J., MacLean P.S., Ir D., Robertson C.E., Orlicky D.J., McManaman J.L. Perilipin-2 modulates lipid absorption and microbiome responses in the mouse intestine. PLoS One. 2015;10:e0131944. doi: 10.1371/journal.pone.0131944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang L.-J., Wang C., Yuan Y., Wang H., Wu J., Liu F., Li L., Gao X., Zhao Y.-L., Hu P.-Z., Li P., Ye J. Cideb facilitates the lipidation of chylomicrons in the small intestine. J Lipid Res. 2014;55:1279–1287. doi: 10.1194/jlr.M046482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khalifeh-Soltani A., Gupta D., Ha A., Iqbal J., Hussain M., Podolsky M.J., Atabai K. Mfge8 regulates enterocyte lipid storage by promoting enterocyte triglyceride hydrolase activity. JCI Insight. 2016;1:e87418. doi: 10.1172/jci.insight.87418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hashidate-Yoshida T., Harayama T., Hishikawa D., Morimoto R., Hamano F., Tokuoka S.M., Eto M., Tamura-Nakano M., Yanobu-Takanashi R., Mukumoto Y., Kiyonari H., Okamura T., Kita Y., Shindou H., Shimizu T. Fatty acid remodeling by LPCAT3 enriches arachidonate in phospholipid membranes and regulates triglyceride transport. Elife. 2015;4:e06328. doi: 10.7554/eLife.06328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li Z., Jiang H., Ding T., Lou C., Bui H.H., Kuo M.-S., Jiang X.-C. Deficiency in lysophosphatidylcholine acyltransferase 3 reduces plasma levels of lipids by reducing lipid absorption in mice. Gastroenterology. 2015;149:1519–1529. doi: 10.1053/j.gastro.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rong X., Wang B., Dunham M.M., Hedde P.N., Wong J.S., Gratton E., Young S.G., Ford D.A., Tontonoz P. Lpcat3-dependent production of arachidonoyl phospholipids is a key determinant of triglyceride secretion. Elife. 2015;4:e06557. doi: 10.7554/eLife.06557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.O’Doherty P.J., Kakis G., Kuksis A. Role of luminal lecithin in intestinal fat absorption. Lipids. 1973;8:249–255. doi: 10.1007/BF02531899. [DOI] [PubMed] [Google Scholar]
- 59.Werner A., Havinga R., Perton F., Kuipers F., Verkade H.J. Lymphatic chylomicron size is inversely related to biliary phospholipid secretion in mice. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1177–G1185. doi: 10.1152/ajpgi.00127.2005. [DOI] [PubMed] [Google Scholar]
- 60.O’Hare E.A., Yang R., Yerges-Armstrong L.M., Sreenivasan U., McFarland R., Leitch C.C., Wilson M.H., Narina S., Gorden A., Ryan K.A., Shuldiner A.R., Farber S.A., Wood G.C., Still C.D., Gerhard G.S., Robishaw J.D., Sztalryd C., Zaghloul N.A. TM6SF2 rs58542926 impacts lipid processing in liver and small intestine. Hepatology. 2017;65:1526–1542. doi: 10.1002/hep.29021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takahara E., Yuasa H., Nishida M., Mantani Y., Udayanga K.G.S., Qi W.-M., Takeuchi T., Yokoyama T., Hoshi N., Kitagawa H. Immunohistochemical and histoplanimetrical study on the endothelial receptor involved in transportation of minute chylomicrons into subepithelial portal blood in intestinal villi of the rat jejunum. J Vet Med Sci. 2015;77:387–393. doi: 10.1292/jvms.14-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roulis M., Flavell R.A. Fibroblasts and myofibroblasts of the intestinal lamina propria in physiology and disease. Differ Res Biol Divers. 2016;92:116–131. doi: 10.1016/j.diff.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 63.Steenbergen J.M., Lash J.M., Bohlen H.G. Role of a lymphatic system in glucose absorption and the accompanying microvascular hyperemia. Am J Physiol. 1994;267:G529–G535. doi: 10.1152/ajpgi.1994.267.4.G529. [DOI] [PubMed] [Google Scholar]
- 64.Bohlen H.G. Mechanism of increased vessel wall nitric oxide concentrations during intestinal absorption. Am J Physiol. 1998;275:H542–H550. doi: 10.1152/ajpheart.1998.275.2.H542. [DOI] [PubMed] [Google Scholar]
- 65.Matheson P.J., Li N., Harris P.D., Zakaria E.R., Garrison R.N. Glucose-induced intestinal vasodilation via adenosine A1 receptors requires nitric oxide but not K(+)(ATP) channels. J Surg Res. 2011;168:179–187. doi: 10.1016/j.jss.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Palacios-Callender M., Hollis V., Mitchison M., Frakich N., Unitt D., Moncada S. Cytochrome c oxidase regulates endogenous nitric oxide availability in respiring cells: a possible explanation for hypoxic vasodilation. Proc Natl Acad Sci U S A. 2007;104:18508–18513. doi: 10.1073/pnas.0709440104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vanner S., Surprenant A. Neural reflexes controlling intestinal microcirculation. Am J Physiol. 1996;271:G223–G230. doi: 10.1152/ajpgi.1996.271.2.G223. [DOI] [PubMed] [Google Scholar]
- 68.Espey M.G. Role of oxygen gradients in shaping redox relationships between the human intestine and its microbiota. Free Radic Biol Med. 2013;55:130–140. doi: 10.1016/j.freeradbiomed.2012.10.554. [DOI] [PubMed] [Google Scholar]
- 69.Zheng L., Kelly C.J., Colgan S.P. Physiologic hypoxia and oxygen homeostasis in the healthy intestine. A review in the theme: cellular responses to hypoxia. Am J Physiol Cell Physiol. 2015;309:C350–C360. doi: 10.1152/ajpcell.00191.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Takahara E.-I., Mantani Y., Udayanga K.G.S., Qi W.-M., Tanida T., Takeuchi T., Yokoyama T., Hoshi N., Kitagawa H. Ultrastructural demonstration of the absorption and transportation of minute chylomicrons by subepithelial blood capillaries in rat jejunal villi. J Vet Med Sci. 2013;75:1563–1569. doi: 10.1292/jvms.13-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bernier-Latmani J., Petrova T.V. Intestinal lymphatic vasculature: structure, mechanisms and functions. Nat Rev Gastroenterol Hepatol. 2017;14:510–526. doi: 10.1038/nrgastro.2017.79. [DOI] [PubMed] [Google Scholar]
- 72.Cifarelli V., Eichmann A. Intestinal lymphatic system: functions and metabolic implications. Cell Mol Gastroenterol Hepatol. 2018 doi: 10.1016/j.jcmgh.2018.12.002. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dixon J.B. Mechanisms of chylomicron uptake into lacteals. Ann N Y Acad Sci. 2010;1207(Suppl 1):E52–E57. doi: 10.1111/j.1749-6632.2010.05716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Van Dyck F., Braem C.V., Chen Z., Declercq J., Deckers R., Kim B.-M., Ito S., Wu M.K., Cohen D.E., Dewerchin M., Derua R., Waelkens E., Fiette L., Roebroek A., Schuit F., Van de Ven W.J.M., Shivdasani R.A. Loss of the PlagL2 transcription factor affects lacteal uptake of chylomicrons. Cell Metab. 2007;6:406–413. doi: 10.1016/j.cmet.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 75.Zhang F., Zarkada G., Han J. Lacteal junction zippering protects against diet-induced obesity. Science. 2018;361:599–603. doi: 10.1126/science.aap9331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kassis T., Yarlagadda S.C., Kohan A.B., Tso P., Breedveld V., Dixon J.B. Postprandial lymphatic pump function after a high-fat meal: a characterization of contractility, flow, and viscosity. Am J Physiol Gastrointest Liver Physiol. 2016;310:G776–G789. doi: 10.1152/ajpgi.00318.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Scallan J.P., Zawieja S.D., Castorena-Gonzalez J.A., Davis M.J. Lymphatic pumping: mechanics, mechanisms and malfunction. J Physiol. 2016;594:5749–5768. doi: 10.1113/JP272088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Choe K., Jang J.Y., Park I., Kim Y., Ahn S., Park D.-Y., Hong Y.-K., Alitalo K., Koh G.Y., Kim P. Intravital imaging of intestinal lacteals unveils lipid drainage through contractility. J Clin Invest. 2015;125:4042–4052. doi: 10.1172/JCI76509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zawieja S.D., Wang W., Wu X., Nepiyushchikh Z.V., Zawieja D.C., Muthuchamy M. Impairments in the intrinsic contractility of mesenteric collecting lymphatics in a rat model of metabolic syndrome. Am J Physiol Heart Circ Physiol. 2012;302:H643–H653. doi: 10.1152/ajpheart.00606.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chakraborty S., Zawieja S., Wang W., Zawieja D.C., Muthuchamy M. Lymphatic system: a vital link between metabolic syndrome and inflammation. Ann N Y Acad Sci. 2010;1207(Suppl 1):E94–E102. doi: 10.1111/j.1749-6632.2010.05752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alitalo K. The lymphatic vasculature in disease. Nat Med. 2011;17:1371–1380. doi: 10.1038/nm.2545. [DOI] [PubMed] [Google Scholar]
- 82.Nurmi H., Saharinen P., Zarkada G., Zheng W., Robciuc M.R., Alitalo K. VEGF-C is required for intestinal lymphatic vessel maintenance and lipid absorption. EMBO Mol Med. 2015;7:1418–1425. doi: 10.15252/emmm.201505731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bui H.M., Enis D., Robciuc M.R., Nurmi H.J., Cohen J., Chen M., Yang Y., Dhillon V., Johnson K., Zhang H., Kirkpatrick R., Traxler E., Anisimov A., Alitalo K., Kahn M.L. Proteolytic activation defines distinct lymphangiogenic mechanisms for VEGFC and VEGFD. J Clin Invest. 2016;126:2167–2180. doi: 10.1172/JCI83967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim K.E., Sung H.-K., Koh G.Y. Lymphatic development in mouse small intestine. Dev Dyn. 2007;236:2020–2025. doi: 10.1002/dvdy.21200. [DOI] [PubMed] [Google Scholar]
- 85.Bernier-Latmani J., Cisarovsky C., Demir C.S., Bruand M., Jaquet M., Davanture S., Ragusa S., Siegert S., Dormond O., Benedito R., Radtke F., Luther S.A., Petrova T.V. DLL4 promotes continuous adult intestinal lacteal regeneration and dietary fat transport. J Clin Invest. 2015;125:4572–4586. doi: 10.1172/JCI82045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gogineni A., Caunt M., Crow A., Lee C.V., Fuh G., van Bruggen N., Ye W., Weimer R.M. Inhibition of VEGF-C modulates distal lymphatic remodeling and secondary metastasis. PLoS One. 2013;8:e68755. doi: 10.1371/journal.pone.0068755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hosoyamada Y., Sakai T. Structural and mechanical architecture of the intestinal villi and crypts in the rat intestine: integrative reevaluation from ultrastructural analysis. Anat Embryol (Berl) 2005;210:1–12. doi: 10.1007/s00429-005-0011-y. [DOI] [PubMed] [Google Scholar]
- 88.Hoopes S.L., Willcockson H.H., Caron K.M. Characteristics of multi-organ lymphangiectasia resulting from temporal deletion of calcitonin receptor-like receptor in adult mice. PLoS One. 2012;7:e45261. doi: 10.1371/journal.pone.0045261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Davis R.B., Kechele D.O., Blakeney E.S., Pawlak J.B., Caron K.M. Lymphatic deletion of calcitonin receptor-like receptor exacerbates intestinal inflammation. JCI Insight. 2017;2:e92465. doi: 10.1172/jci.insight.92465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ahrén B., Holst J.J. The cephalic insulin response to meal ingestion in humans is dependent on both cholinergic and noncholinergic mechanisms and is important for postprandial glycemia. Diabetes. 2001;50:1030–1038. doi: 10.2337/diabetes.50.5.1030. [DOI] [PubMed] [Google Scholar]
- 91.Simonian H.P., Kresge K.M., Boden G.H., Parkman H.P. Differential effects of sham feeding and meal ingestion on ghrelin and pancreatic polypeptide levels: evidence for vagal efferent stimulation mediating ghrelin release. Neurogastroenterol Motil. 2005;17:348–354. doi: 10.1111/j.1365-2982.2004.00634.x. [DOI] [PubMed] [Google Scholar]
- 92.Veedfald S., Plamboeck A., Deacon C.F., Hartmann B., Knop F.K., Vilsbøll T., Holst J.J. Cephalic phase secretion of insulin and other enteropancreatic hormones in humans. Am J Physiol Gastrointest Liver Physiol. 2016;310:G43–G51. doi: 10.1152/ajpgi.00222.2015. [DOI] [PubMed] [Google Scholar]
- 93.Robertson M.D., Mason A.O., Frayn K.N. Timing of vagal stimulation affects postprandial lipid metabolism in humans. Am J Clin Nutr. 2002;76:71–77. doi: 10.1093/ajcn/76.1.71. [DOI] [PubMed] [Google Scholar]
- 94.Mattes R.D. Brief oral stimulation, but especially oral fat exposure, elevates serum triglycerides in humans. Am J Physiol Gastrointest Liver Physiol. 2009;296:G365–G371. doi: 10.1152/ajpgi.90591.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mattes R.D. Is there a fatty acid taste? Annu Rev Nutr. 2009;29:305–327. doi: 10.1146/annurev-nutr-080508-141108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Laugerette F., Passilly-Degrace P., Patris B., Niot I., Febbraio M., Montmayeur J.-P., Besnard P. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J Clin Invest. 2005;115:3177–3184. doi: 10.1172/JCI25299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gaillard D., Laugerette F., Darcel N., El-Yassimi A., Passilly-Degrace P., Hichami A., Khan N.A., Montmayeur J.-P., Besnard P. The gustatory pathway is involved in CD36-mediated orosensory perception of long-chain fatty acids in the mouse. FASEB J. 2008;22:1458–1468. doi: 10.1096/fj.07-8415com. [DOI] [PubMed] [Google Scholar]
- 98.El-Yassimi A., Hichami A., Besnard P., Khan N.A. Linoleic acid induces calcium signaling, Src kinase phosphorylation, and neurotransmitter release in mouse CD36-positive gustatory cells. J Biol Chem. 2008;283:12949–12959. doi: 10.1074/jbc.M707478200. [DOI] [PubMed] [Google Scholar]
- 99.Keller K.L., Liang L.C.H., Sakimura J., May D., van Belle C., Breen C., Driggin E., Tepper B.J., Lanzano P.C., Deng L., Chung W.K. Common variants in the CD36 gene are associated with oral fat perception, fat preferences, and obesity in African Americans. Obesity (Silver Spring) 2012;20:1066–1073. doi: 10.1038/oby.2011.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ahmed M.Y., Al-Khayat A., Al-Murshedi F., Al-Futaisi A., Chioza B.A., Pedro Fernandez-Murray J., Self J.E., Salter C.G., Harlalka G.V., Rawlins L.E., Al-Zuhaibi S., Al-Azri F., Al-Rashdi F., Cazenave-Gassiot A., Wenk M.R., Al-Salmi F., Patton M.A., Silver D.L., Baple E.L., McMaster C.R., Crosby A.H. A mutation of EPT1 (SELENOI) underlies a new disorder of Kennedy pathway phospholipid biosynthesis. Brain J Neurol. 2017;140:547–554. doi: 10.1093/brain/aww318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Payne F., Lim K., Girousse A., Brown R.J., Kory N., Robbins A., Xue Y., Sleigh A., Cochran E., Adams C., Dev Borman A., Russel-Jones D., Gorden P., Semple R.K., Saudek V., O’Rahilly S., Walther T.C., Barroso I., Savage D.B. Mutations disrupting the Kennedy phosphatidylcholine pathway in humans with congenital lipodystrophy and fatty liver disease. Proc Natl Acad Sci U S A. 2014;111:8901–8906. doi: 10.1073/pnas.1408523111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fagerberg L., Hallström B.M., Oksvold P., Kampf C., Djureinovic D., Odeberg J., Habuka M., Tahmasebpoor S., Danielsson A., Edlund K., Asplund A., Sjöstedt E., Lundberg E., Szigyarto C.A.-K., Skogs M., Takanen J.O., Berling H., Tegel H., Mulder J., Nilsson P., Schwenk J.M., Lindskog C., Danielsson F., Mardinoglu A., Sivertsson A., von Feilitzen K., Forsberg M., Zwahlen M., Olsson I., Navani S., Huss M., Nielsen J., Ponten F., Uhlén M. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteomics. 2014;13:397–406. doi: 10.1074/mcp.M113.035600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Persson B., Kallberg Y., Bray J.E., Bruford E., Dellaporta S.L., Favia A.D., Duarte R.G., Jörnvall H., Kavanagh K.L., Kedishvili N., Kisiela M., Maser E., Mindnich R., Orchard S., Penning T.M., Thornton J.M., Adamski J., Oppermann U. The SDR (short-chain dehydrogenase/reductase and related enzymes) nomenclature initiative. Chem Biol Interact. 2009;178:94–98. doi: 10.1016/j.cbi.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Oppermann U.C., Filling C., Jörnvall H. Forms and functions of human SDR enzymes. Chem Biol Interact. 2001;130–132:699–705. doi: 10.1016/s0009-2797(00)00301-x. [DOI] [PubMed] [Google Scholar]
- 105.Lee J.S. Luminal and plasma glucose concentrations on intestinal fluid absorption and lymph flow. Am J Physiol. 1987;252:G568–G573. doi: 10.1152/ajpgi.1987.252.4.G568. [DOI] [PubMed] [Google Scholar]
- 106.Bohlen H.G., Unthank J.L. Rat intestinal lymph osmolarity during glucose and oleic acid absorption. Am J Physiol. 1989;257:G438–G446. doi: 10.1152/ajpgi.1989.257.3.G438. [DOI] [PubMed] [Google Scholar]
- 107.Scott E.M., Greenwood J.P., Vacca G., Stoker J.B., Gilbey S.G., Mary D.A.S.G. Carbohydrate ingestion, with transient endogenous insulinaemia, produces both sympathetic activation and vasodilatation in normal humans. Clin Sci (Lond) 2002;102:523–529. [PubMed] [Google Scholar]
- 108.Westberg-Rasmussen S., Starup-Linde J., Hermansen K., Holst J.J., Hartmann B., Vestergaard P., Gregersen S. Differential impact of glucose administered intravenously or orally on bone turnover markers in healthy male subjects. Bone. 2017;97:261–266. doi: 10.1016/j.bone.2017.01.027. [DOI] [PubMed] [Google Scholar]
- 109.Hartmann B., Harr M.B., Jeppesen P.B., Wojdemann M., Deacon C.F., Mortensen P.B., Holst J.J. In vivo and in vitro degradation of glucagon-like peptide-2 in humans. J Clin Endocrinol Metab. 2000;85:2884–2888. doi: 10.1210/jcem.85.8.6717. [DOI] [PubMed] [Google Scholar]
- 110.Hsieh J., Longuet C., Maida A., Bahrami J., Xu E., Baker C.L., Brubaker P.L., Drucker D.J., Adeli K. Glucagon-like peptide-2 increases intestinal lipid absorption and chylomicron production via CD36. Gastroenterology. 2009;137:997–1005. doi: 10.1053/j.gastro.2009.05.051. 1005.e1–4. [DOI] [PubMed] [Google Scholar]
- 111.Meier J.J., Nauck M.A., Pott A., Heinze K., Goetze O., Bulut K., Schmidt W.E., Gallwitz B., Holst J.J. Glucagon-like peptide 2 stimulates glucagon secretion, enhances lipid absorption, and inhibits gastric acid secretion in humans. Gastroenterology. 2006;130:44–54. doi: 10.1053/j.gastro.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 112.El-Jamal N., Erdual E., Neunlist M., Koriche D., Dubuquoy C., Maggiotto F., Chevalier J., Berrebi D., Dubuquoy L., Boulanger E., Cortot A., Desreumaux P. Glugacon-like peptide-2: broad receptor expression, limited therapeutic effect on intestinal inflammation and novel role in liver regeneration. Am J Physiol Gastrointest Liver Physiol. 2014;307:G274–G285. doi: 10.1152/ajpgi.00389.2012. [DOI] [PubMed] [Google Scholar]
- 113.Pedersen J., Pedersen N.B., Brix S.W., Grunddal K.V., Rosenkilde M.M., Hartmann B., Ørskov C., Poulsen S.S., Holst J.J. The glucagon-like peptide 2 receptor is expressed in enteric neurons and not in the epithelium of the intestine. Peptides. 2015;67:20–28. doi: 10.1016/j.peptides.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 114.Yusta B., Huang L., Munroe D., Wolff G., Fantaske R., Sharma S., Demchyshyn L., Asa S.L., Drucker D.J. Enteroendocrine localization of GLP-2 receptor expression in humans and rodents. Gastroenterology. 2000;119:744–755. doi: 10.1053/gast.2000.16489. [DOI] [PubMed] [Google Scholar]
- 115.Guan X., Karpen H.E., Stephens J., Bukowski J.T., Niu S., Zhang G., Stoll B., Finegold M.J., Holst J.J., Hadsell D., Nichols B.L., Burrin D.G. GLP-2 receptor localizes to enteric neurons and endocrine cells expressing vasoactive peptides and mediates increased blood flow. Gastroenterology. 2006;130:150–164. doi: 10.1053/j.gastro.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 116.Guan X., Shi X., Li X., Chang B., Wang Y., Li D., Chan L. GLP-2 receptor in POMC neurons suppresses feeding behavior and gastric motility. Am J Physiol Endocrinol Metab. 2012;303:E853–E864. doi: 10.1152/ajpendo.00245.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wismann P., Barkholt P., Secher T., Vrang N., Hansen H.B., Jeppesen P.B., Baggio L.L., Koehler J.A., Drucker D.J., Sandoval D.A., Jelsing J. The endogenous preproglucagon system is not essential for gut growth homeostasis in mice. Mol Metab. 2017;6:681–692. doi: 10.1016/j.molmet.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ørskov C., Hartmann B., Poulsen S.S., Thulesen J., Hare K.J., Holst J.J. GLP-2 stimulates colonic growth via KGF, released by subepithelial myofibroblasts with GLP-2 receptors. Regul Pept. 2005;124:105–112. doi: 10.1016/j.regpep.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 119.Furness J.B. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9:286–294. doi: 10.1038/nrgastro.2012.32. [DOI] [PubMed] [Google Scholar]
- 120.Cinci L., Faussone-Pellegrini M.S., Rotondo A., Mulè F., Vannucchi M.G. GLP-2 receptor expression in excitatory and inhibitory enteric neurons and its role in mouse duodenum contractility. Neurogastroenterol Motil. 2011;23:e383–e392. doi: 10.1111/j.1365-2982.2011.01750.x. [DOI] [PubMed] [Google Scholar]
- 121.Sigalet D.L., Wallace L., De Heuval E., Sharkey K.A. The effects of glucagon-like peptide 2 on enteric neurons in intestinal inflammation. Neurogastroenterol Motil. 2010;22 doi: 10.1111/j.1365-2982.2010.01585.x. 1318-e350. [DOI] [PubMed] [Google Scholar]
- 122.de Heuvel E., Wallace L., Sharkey K.A., Sigalet D.L. Glucagon-like peptide 2 induces vasoactive intestinal polypeptide expression in enteric neurons via phophatidylinositol 3-kinase-γ signaling. Am J Physiol Endocrinol Metab. 2012;303:E994–E1005. doi: 10.1152/ajpendo.00291.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bremholm L., Hornum M., Andersen U.B., Hartmann B., Holst J.J., Jeppesen P.B. The effect of glucagon-like peptide-2 on mesenteric blood flow and cardiac parameters in end-jejunostomy short bowel patients. Regul Pept. 2011;168:32–38. doi: 10.1016/j.regpep.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 124.Bremholm L., Hornum M., Andersen U.B., Holst J.J. The effect of glucagon-like peptide-2 on arterial blood flow and cardiac parameters. Regul Pept. 2010;159:67–71. doi: 10.1016/j.regpep.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 125.Guan X., Stoll B., Lu X., Tappenden K.A., Holst J.J., Hartmann B., Burrin D.G. GLP-2-mediated up-regulation of intestinal blood flow and glucose uptake is nitric oxide-dependent in TPN-fed piglets 1. Gastroenterology. 2003;125:136–147. doi: 10.1016/s0016-5085(03)00667-x. [DOI] [PubMed] [Google Scholar]
- 126.Deniz M., Bozkurt A., Kurtel H. Mediators of glucagon-like peptide 2-induced blood flow: responses in different vascular sites. Regul Pept. 2007;142:7–15. doi: 10.1016/j.regpep.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 127.Hsieh J., Trajcevski K.E., Farr S.L., Baker C.L., Lake E.J., Taher J., Iqbal J., Hussain M.M., Adeli K. Glucagon-like peptide 2 (GLP-2) stimulates postprandial chylomicron production and postabsorptive release of intestinal triglyceride storage pools via induction of nitric oxide signaling in male hamsters and mice. Endocrinology. 2015;156:3538–3547. doi: 10.1210/EN.2015-1110. [DOI] [PubMed] [Google Scholar]
- 128.Xiao C., Stahel P., Dash S., Morgantini C., Lewis G.F. Investigation of the role of enteric blood flow in mediating GLP-2 stimulation of triglyceride mobilization and chylomicron secretion in the human intestine. Atheroscler Suppl. 2018;32:29. [Google Scholar]
- 129.Bulut K., Pennartz C., Felderbauer P., Meier J.J., Banasch M., Bulut D., Schmitz F., Schmidt W.E., Hoffmann P. Glucagon like peptide-2 induces intestinal restitution through VEGF release from subepithelial myofibroblasts. Eur J Pharmacol. 2008;578:279–285. doi: 10.1016/j.ejphar.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 130.Rask-Madsen C., King G.L. Differential regulation of VEGF signaling by PKC-alpha and PKC-epsilon in endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28:919–924. doi: 10.1161/ATVBAHA.108.162842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Guan X. The CNS glucagon-like peptide-2 receptor in the control of energy balance and glucose homeostasis. Am J Physiol Regul Integr Comp Physiol. 2014;307:R585–R596. doi: 10.1152/ajpregu.00096.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shi X., Zhou F., Li X., Chang B., Li D., Wang Y., Tong Q., Xu Y., Fukuda M., Zhao J.J., Li D., Burrin D.G., Chan L., Guan X. Central GLP-2 enhances hepatic insulin sensitivity via activating PI3K signaling in POMC neurons. Cell Metab. 2013;18:86–98. doi: 10.1016/j.cmet.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Browning K.N., Travagli R.A. Central nervous system control of gastrointestinal motility and secretion and modulation of gastrointestinal functions. Compr Physiol. 2014;4:1339–1368. doi: 10.1002/cphy.c130055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Farr S., Taher J., Adeli K. Central nervous system regulation of intestinal lipid and lipoprotein metabolism. Curr Opin Lipidol. 2016;27:1–7. doi: 10.1097/MOL.0000000000000254. [DOI] [PubMed] [Google Scholar]