Abstract
Context
Astaxanthin has been shown to provide important health benefits, such as functioning as an anti-inflammatory, antioxidant, anticancer, and cardioprotective agent. Astaxanthin is a lipid-soluble molecule with low oral bioavailability, which limits its therapeutic potential. The low oral bioavailability is due to dissolution limitations in the gastrointestinal fluids.
Objective
The objective of this study was to compare the relative bioavailability of a proprietary sustained-release formulation of astaxanthin (astaxanthin-SR)—a micronized dispersion of astaxanthin oil that is 2.5% astaxanthin in a sustained-release matrix—with that of an unformulated astaxanthin oil containing 10% astaxanthin.
Design
A dissolution study compared the solubility of formulated and unformulated astaxanthin oils. The research team also performed a single-dose, 24-h crossover, uptake study.
Setting
The dissolution study took place at BioActives (Worcester, MA, USA). The single-dose study was done at the MAZE Laboratories (Purchase, NY, USA).
Participants
Six healthy male and female volunteers aged 21 to 66 y took part in the single-dose study. The participants were people from the community.
Intervention
That proprietary formulation is a free-flowing paste that is able to form a stable dispersion in water and achieve sustained-release when presented in a capsule form. For the samples used in the dissolution study, hard gelatin capsules were filled either with the unformulated astaxanthin oil or with the proprietary astaxanthin-SR formulation, both with the equivalent of 10 mg of astaxanthin. For the single-dose study, participants received a 60-mg dose of astaxanthin in each form, as capsules, with a 15-d washout period between the 2 doses. The astaxanthin-SR capsules were administered after breakfast, and blood samples were drawn at 3, 8, 10, and 24 h postintervention. After a 15-d washout period, the protocol was repeated with astaxanthin oil capsules.
Outcome Measures
For the dissolution study, the astaxanthin was quantified using spectrophotometry. For the single-dose study, plasma astaxanthin was quantified using reverse-phase high-performance liquid chromatography equipped with an ultraviolet-visible detector and a Phenomenex Synergy Hydro-RP column 150 × 4.6 mm, 4 μm (Phenomenex, Torrance, CA, USA) at room temperature.
Results
The dissolution study indicated that the astaxanthin-SR formulation formed a stable dispersion in the simulated gastric and intestinal fluids. The formulation also showed greater dissolution for 12 h at all points tested, compared with the astaxanthin oil, which showed no dissolution during the same period of 12 h. The results of the single-dose uptake study indicated that the astaxanthin-SR formulation was 3.6 times more bioavailable than astaxanthin oil, with P < .0005 in a paired t test. In addition, all participants showed uptake from the sustained-release formulation.
Conclusions
The formulation of astaxanthin oil in a sustained-release matrix significantly improved the absorption of astaxanthin. The formulation also reduced interindividual variations in absorption. Participants who were poor absorbers from the unformulated astaxanthin oil, showed higher absorption with the astaxanthin-SR formulation.
Astaxanthin—3,3′-dihydroxy-β, β′-carotene-4,4′-dione—is a naturally occurring carotenoid belonging to the xanthophyll group. It is synthesized by microorganisms, plants, and algae, but animals lack the ability to synthesize carotenoids. Astaxanthin is widely distributed in marine organisms by ingestion and bioaccumulation in the food web.
Astaxanthin is reported to have diverse health benefits. It is a potent antioxidant as compared to β-carotene, vitamin C, vitamin E, lutein, lycopene, and catechins in in vitro studies.1 In human studies, astaxanthin has been reported to reduce oxidative stress markers in the blood and improve antioxidant markers.2,3
Astaxanthin supplementation has been reported to decrease inflammation and enhance immune response in humans.4 Cell-based and animal studies have suggested that astaxanthin has many therapeutic and preventive benefits, such as protecting the skin from ultraviolet radiation,5 improving cardiovascular health,6 providing anticancer and antidiabetic benefits, and protecting the eyes.7 Katagiri et al8 reported improved cognitive scores in older individuals with age-related forgetfulness. Astaxanthin crosses the blood-brain barrier and has the potential to reduce or prevent the conditions associated with brain aging.9
Commercially available astaxanthin-enriched extract—astaxanthin oil that is 10% astaxanthin—contains mainly esters of astaxanthin from the algae Haematococcus pluvialis and is widely used in the dietary supplement industry.
Astaxanthin is a lipid-soluble molecule with low oral bioavailability, which limits its therapeutic potential. The low oral bioavailability is due to dissolution limitations in the gastrointestinal fluids. The uptake of carotenoids depends on their solubility in dietary fats and on the secretion of bile acids to form an emulsion with the gastrointestinal fluid.
Following partial digestion of lipids, carotenoids are solubilized in the mixed micelles, which contain other components, such as bile salts, phospholipids, fatty acids, and cholesterol. The micelle-containing carotenoids are passively absorbed by the intestinal cells. A strong indication also exists for receptor-mediated uptake of carotenoids by the enterocytes. The interindividual variations in bioavailability are due to many factors, such as dietary components, timing of ingestion, amount of dietary fats, variations in bile acid secretion, and dissolution limitations in the gastrointestinal fluids.10,11
To increase its solubility, the use of nanodispersions, nanoemulsion formulations, and microencapsulation in hydrophilic carriers has been reported.12-14 Such formulations involve expensive equipment and show low concentrations of active compounds and poor stability of the nanoparticles, and the end product becomes economically unviable for the supplement industry.
In a human study, Odeberg et al15 reported improved oral bioavailability for formulations of spray-dried Haematococcus biomass in a combination of lipids and surfactants. The lower concentration of astaxanthin in the biomass and difficulty of formulating limits the use of such formulations in dosage forms.
The objective of the current study was to compare the relative bioavailability of a proprietary formulation, astaxanthin-SR—a micronized dispersion of astaxanthin oil that is 2.5% astaxanthin in a sustained-release matrix—with that of an unformulated astaxanthin oil containing 10% astaxanthin.
Materials and Methods
Materials
Astaxanthin oil (H pluvialis extract containing 10% astaxanthin as mono-and diesters) was provided by Maypro Industries (Purchase, NY, USA). High-performance liquid chromatography (HPLC) grade solvents and other chemicals were obtained from VWR International (Radnor, PA, USA). Standard free astaxanthin (96% purity by HPLC) was obtained from Chromadex (Irvine, CA, USA).
Methods: Dissolution Test
The dissolution study took place at BioActives LLC (Worcester, MA, USA). The study was performed using the Varian 7020 dissolution tester with the basket configuration at 37°C, 100 RPM. The samples equivalent to 10 mg of astaxanthin filled in hard gelatin capsules were used for the study. The capsules were introduced into 750 mL of 0.1N HCl (simulated gastric fluid without enzymes, SGF) maintained at 37°C. At the end of 1 and 2 hours, 3 mL of the aliquot was withdrawn and filtered through a 10-μ syringe filter. The removed volume was replaced each time with fresh medium. At the end of 2 hours, the pH of the medium was adjusted to 6.8 (simulated intestinal fluid without enzymes, SIF) with 195 mL of 0.2M tribasic sodium phosphate solution equilibrated to 37°C. Polysorbate 80 dissolved in 55 mL of water was added to a concentration of 0.25% to simulate intestinal fluid. Aliquots of 3 mL were withdrawn at 4, 6, 9, and 12 hours for analysis and the removed volume was replaced with fresh medium each time.
Methods: Clinical Study: Participants
The single-dose, 24-hour crossover, uptake study took place at the MAZE Laboratories (Purchase, NY, USA). The study was reviewed by Sterling Institutional Review Board (Atlanta, GA, USA). Written informed consent was obtained from all the participants.
The participants were people from the community recruited by advertising in local papers. Six healthy male and female volunteers between the ages of 21 and 66 years who had not been consuming astaxanthin supplements were selected from 10 volunteers for the study. The participants were not using any other medications.
Potential participants were excluded if they (1) had known malabsorption or maldigestion, diabetes, hypertension, gall bladder disease, or any condition that the principal investigator believed might put the participant at undue risk; (2) had a body mass index (BMI) <18 kg/m2 or >30 kg/m2; (3) took medication for a chronic condition; (4) had a history or current abuse of drugs, medication, or alcohol, or intake of more than 2 alcoholic beverages per day; (5) had a known hypersensitivity to the study’s materials or to any ingredient in their preparation; (6) were pregnant or lactating; or (7) had participated in another clinical trial within the 4 weeks prior to the study’s start or would be concurrently participating in another clinical trial.
Intervention
The single-dose bioavailability study compared the 2 forms of astaxanthin, both in capsules: (1) a commercially available unformulated astaxanthin oil standardized to contain 10% astaxanthin and (2) astaxanthin-SR, a micronized sustained release formulation containing 2.5% astaxanthin. The participants received a 60-mg dose of astaxanthin in each form, as capsules, with a 15-day washout period between the 2 doses.
Procedures
The participants were instructed not to consume any astaxanthin-containing foods or supplements for at least 1 week prior to the study. They were also instructed not to consume any food for at least 12 hours prior to the blood draw at baseline. Following the overnight fasting, 7 mL of blood was collected in ethylenediaminetetraacetic acid (EDTA) tubes to permit establishment of a baseline value.
On the day of the study, a standard breakfast; a lunch of approximately 500 kcal with 15% protein, 30% fat, and 55% carbohydrate; and a snack were offered to the participants. Astaxanthin-SR capsules were administered after breakfast. Blood samples were drawn at 3, 8, 10, and 24 hours postintervention. The blood samples were stored on ice and protected from light. The plasma was separated by centrifugation within 1 hour of collection and stored at -70°C until analysis.
After a 15-day washout period, the protocol was repeated with the same participants for the capsules of unformulated astaxanthin oil.
Outcome Measures
Dissolution Study
Astaxathin in the aliquots withdrawn at 1, 2, 4, 6, 8, and 12 hours for both samples was quantified by spectrophotometry. For quantification, 0.5 mL of the aliquot was diluted to 3 mL in acetone and the absorbance was measured at 470 nm using a Beckman UV-Vis spectrophotometer (Beckman Coulter Inc, Fullerton, CA, USA). Astaxanthin was quantified using an E1% of 2100 at 470 nm.
Clinical Study
Plasma astaxanthin was extracted from each participant’s blood samples, based on the method described by Khachik et al.16 The astaxanthin was quantified using reverse-phase HPLC, equipped a with ultraviolet-visible detector and a Phenomenex Synergy Hydro-RP column (Phenomenex Inc, Torrance, CA, USA), 150 × 4.6 mm, 4 μm, at room temperature. The samples were eluted using an isocratic mobile phase, consisting of 82% methanol, 10% ethyl acetate, and 8% water. The flow rate was 1 mL/min, and the detection wavelength was 470 nm. The standard astaxanthin from Chromadex was used for the quantification of plasma astaxanthin. The retention time for astaxanthin was 6.2 minutes under those conditions. The standard solution containing a known concentration of astaxanthin was analyzed to obtain the response (ie, the area of the peak corresponding to that concentration). The plasma extracts were analyzed under identical conditions and the response of each sample was compared to the response of the known concentration of the standard to determine the concentration of astaxanthin in the plasma samples.
Statistical Analysis
The amounts of astaxanthin in the plasma for the 2 study samples were determined using the area under the curve (AUC) calculated with the trapezoid rule. The amounts of astaxanthin absorbed by participants for the blood samples drawn at 3, 8, 10, and 24 hours postintervention were compared using the Student t test (repeated measures). Calculations and analysis were made using Prism 4 for Macintosh (GraphPad, La Jolla, CA, USA).
Results
Dissolution Test
Figure 1 presents the dissolution profile of astaxanthin-SR and the astaxanthin oil. Astaxanthin-SR showed a high dissolution at all time points tested. Nearly 40% of the dose was released at an acidic pH by the end of 2 hours. By 12 hours, 85% of the dose was in solution in a micronized form, with a particle size of less than 10 microns. The dissolution profile demonstrated sustained release of the proprietary formulation. The astaxanthin oil showed no dissolution during the same period of 12 hours.
Figure 1.
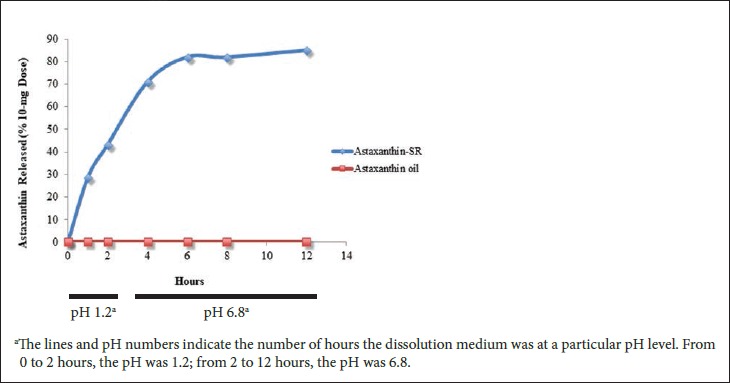
Comparison of the Dissolution Profiles of Astaxanthin-SR and Astaxanthin Oil
Clinical Study
Figure 2 presents the average uptake of astaxanthin from astaxanthin-SR and astaxanthin oil for the 6 participants. Astaxanthin-SR showed higher plasma levels at all time points tested compared to astaxanthin oil, with a Tmax of 8 hours.
Figure 2.
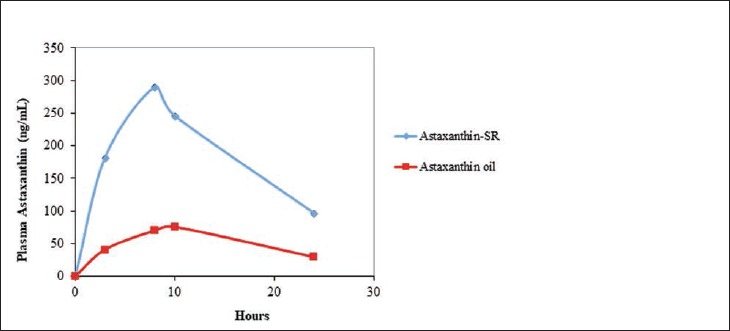
Comparison of the Uptake of Astaxanthin From Astaxanthin-SR and Astaxanthin Oil After a Single Oral Dose
At 24 hours, the plasma level of astaxanthin was 3 times higher for astaxanthin-SR compared to astaxanthin oil. The average area under the curve—AUC 0-24h ng/(mL × h)—for the intervention was 4393 ± 869 and for the control was 1227 ± 1328. The ratio of the AUC of astaxanthin-SR to astaxanthin oil was 3.6, and the statistical results were highly significant at P < .0005.
Figure 3 presents a comparison of the individual AUCs. All participants absorbed astaxanthin from astaxanthin-SR, whereas 3 of the 6 participants showed very poor absorption from astaxanthin oil. Figure 3 presents some insight into the distribution of the uptake of the 2 products by the participants. The data shows a reduction in the variance in the absorption for astaxanthin-SR compared to that of astaxanthin oil. The results also indicate that all the participants absorbed more astaxanthin with astaxanthin-SR as compared with astaxanthin oil, including the highest absorbers.
Figure 3.
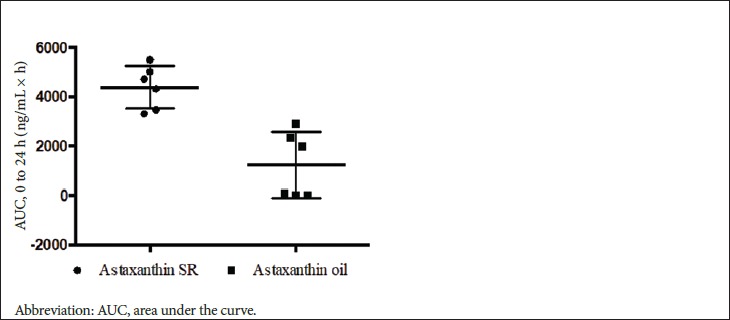
Comparison of the Uptake of Astaxanthin From Astaxanthin-SR and Astaxanthin Oil by Participants
Discussion
The objective of the current study was to compare the relative bioavailability of a proprietary, sustained-release, micronized formulation of astaxanthin, with that of a commercially available, unformulated astaxanthin oil.
In the dissolution study using simulated gastric and intestinal fluids, the proprietary astaxanthin-SR formulation showed a high and sustained dissolution for a period of 12 hours compared with that of the unformulated astaxanthin oil, indicative of sustained release on oral dosing. The proprietary formulation also formed a stable dispersion in the dissolution medium, while the unformulated astaxanthin oil remained immiscible.
The astaxanthin oil used in the study comprised mainly ester forms of astaxanthin, while the plasma showed only free astaxanthin, a result similar to earlier reports by Okada et al17 and Odeberg et al.15
The proprietary astaxanthin-SR formulation showed a significantly higher absorption compared to unformulated astaxanthin oil in the current single-dose uptake study. The bioavailability of astaxanthin from the proprietary astaxanthin formulation was 3.6 times greater than that of the unformulated astaxanthin oil. All participants absorbed astaxanthin from the astaxanthin-SR formulation, whereas 3 of the 6 participants showed very little absorption from the unformulated astaxanthin oil. A reduction occurred in the interindividual variance of the absorption from the proprietary astaxanthin formulation.
The significantly greater bioavailability and reduction in variance could be due to the dissolution, the formation of a stable dispersion of the proprietary formulation, and the sustained release of astaxanthin. The sustained release may have helped to prevent the saturation and precipitation of astaxanthin in the gastrointestinal fluid, thereby facilitating better incorporation into the micelles. Sustained-release micronized proprietary formulations of other lipophilic compounds, such as curcumin and Coenzyme Q10, have demonstrated similar results, ie, greater bioavailability and reduced interindividual variations in absorption.18,19
Previous studies on carotenoids, such as beta carotene and lutein esters, have indicated that the absorption was facilitated by dietary fats.11 Intake of an astaxanthin supplement before or after a meal has also been reported to affect the absorption of astaxanthin.17
In the present study, the astaxanthin dosing occurred after a meal. The study is limited by a small sample size and a single dosing time. Further studies are needed with a larger sample size to confirm the reduction in interindividual variations and determine if the proprietary astaxanthin-SR formulation can overcome the need for dietary fats and timing of dosing. Further studies are also needed to determine the effects of the formulation on the accumulation of astaxanthin in long-term supplementation.
Conclusions
The proprietary formulation of astaxanthin oil in a sustained-release matrix significantly improved the absorption of astaxanthin. The formulation also reduced interindividual variations in absorption. Participants who were poor absorbers from the unformulated astaxanthin oil showed higher absorption with the proprietary astaxanthin-SR formulation.
Biographies
Doddabele Madhavi, PhD, is managing partner
Daniel Kagan, PhD, is managing partner
Supriya Seshadri, PhD, is a consultant at BioActives in Worcester, Massachusetts
References
- 1.Chen JT, Kotani K. Astaxanthin as a potential protector of liver function: A review. J Clin Med Res. 2016;8:701-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karppi J, Rissanen TH, Nyyssonen K, et al. Effects of astaxanthin supplementation on lipid peroxidation. Int J Vitam Nutr Res. 2007;77:3-11. [DOI] [PubMed] [Google Scholar]
- 3.Choi HD, Kim JH, Chang MJ, et al. Effects of astaxanthin on oxidative stress in overweight and obese adults. Phytother Res. 2011;25:1813-1818. [DOI] [PubMed] [Google Scholar]
- 4.Park JS, Chyun JH, Kim YK, et al. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr Metab (London). 2010;7:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hama S, Takahashi K, Inai Y, et al. Protective effects of topical application of a poorly soluble antioxidant astaxanthin liposomal formulation on ultraviolet-induced skin damage. J Pharm Sci. 2012; 101:2909-2916. [DOI] [PubMed] [Google Scholar]
- 6.Fassett RG, Coombes JS. Astaxanthin: A potential therapeutic agent in cardiovascular disease. Mar Drugs. 2011;9:447-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuan JP, Peng J, Yin K, et al. Potential health promoting effects of astaxanthin: A high-value carotenoid mostly from microalgae. Mol Nutr Food Res. 2011;55:150-165. [DOI] [PubMed] [Google Scholar]
- 8.Katagiri M, Satoh A, Tsuji S, et al. Effects of astaxanthin-rich Haematococcus pluvialis extract on cognitive function: A randomized double- blind placebo-controlled study. J Clin Biochem Nutr. 2012;51:102-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grimmig B, Bickford PC, Morganti J, et al. Immunomodulators as therapeutic agents in mitigating the progression of Parkinson’s disease. Brain Sci. 2016;6:2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frommherz L, Bub A, Hummel E. Age related changes of plasma bile acid concentrations in healthy adults-Results from a cross sectional KarMeN study. PLoS ONE. 2016;11:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yonekura L, Nagao A. Intestinal absorption of dietary carotenoids. Mol Nutr Food Res. 2007;51:107-115. [DOI] [PubMed] [Google Scholar]
- 12.Affandi MMM, Julianto T, Mejeed A. Development and stability evaluation of astaxanthin nanoemulsion. Asian J Pharma Clin Res. 2011;4(Suppl 1):143-148. [Google Scholar]
- 13.Anarjan N, Nehdi IN, Tan CP. Protection of astaxanthin in astaxanthin nanodispersions using additional antioxidants. Molecules. 2013;18(7):7699-7710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nalawade P, Gajjar A. Optimization of astaxanthin microencapsulation in hydrophilic carriers using response surface methodology. Arch Pharmacal Res. 2015;1:1. [DOI] [PubMed] [Google Scholar]
- 15.Odberg JM, Lignell A, Pettersson A, et al. Oral bioavailability of the antioxidant astaxanthin in humans is enhanced by incorporation of lipidbased formulations. Eu J Pharm Sci. 2003;19:299-304. [DOI] [PubMed] [Google Scholar]
- 16.Khachik F, Beecher GR, Goli MB, et al. Separation and quantification of carotenoids in human plasma. Methods in Enzymol. 1992;213:205-219. [DOI] [PubMed] [Google Scholar]
- 17.Okada Y, Ishikura M, Maoka T. Bioavailability of astaxanthin in Haematococcus algal extract: The effects of timing of diet and smoking habits. Biosci Biotechnol Biochem. 2009;73:1928-1932. [DOI] [PubMed] [Google Scholar]
- 18.Madhavi D, Kagan D. Bioavailability of a sustained release formulation of curcumin. Integrat Med Clin J. 2014;13:24-30. [PMC free article] [PubMed] [Google Scholar]
- 19.Madhavi D, Kagan D. A study on the bioavailability of a sustained-release coenzyme Q10-β-Cyclodextrin complex. Integrat Med Clin J. 2010;9:20-24. [Google Scholar]


