Figure 3.
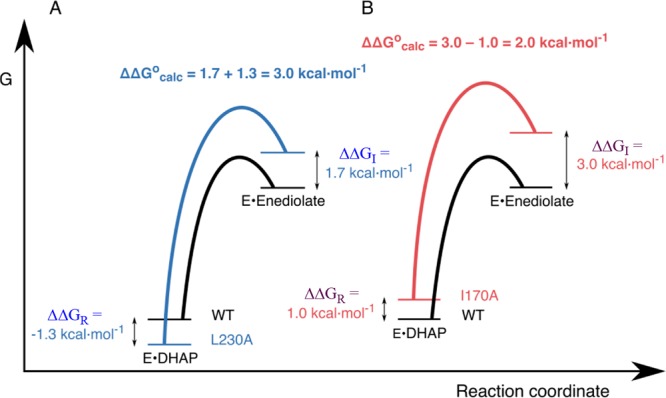
Free energy profiles for deprotonation of enzyme-bound DHAP catalyzed by wild-type and mutant TIMs, which combine results from experimental and computational studies. The diagrams show the effect of these mutations on the stability of the Michaelis complex (ΔΔGR) relative to free TIM determined by experiment, and on the stability of the enediolate intermediate relative to the Michaelis complex (ΔΔGocalc) determined by EVB calculations.15 The effect of these mutations on the stability of the enediolate intermediate relative to free TIM (ΔΔGI) is equal to [(ΔΔGocalc + ΔΔGR]. (A) Profiles for wild-type TIM and the L230A mutant. (B) Profiles for wild-type TIM and the I170A mutant. Reprinted with permission from ref (15). Copyright 2017 American Chemical Society.
