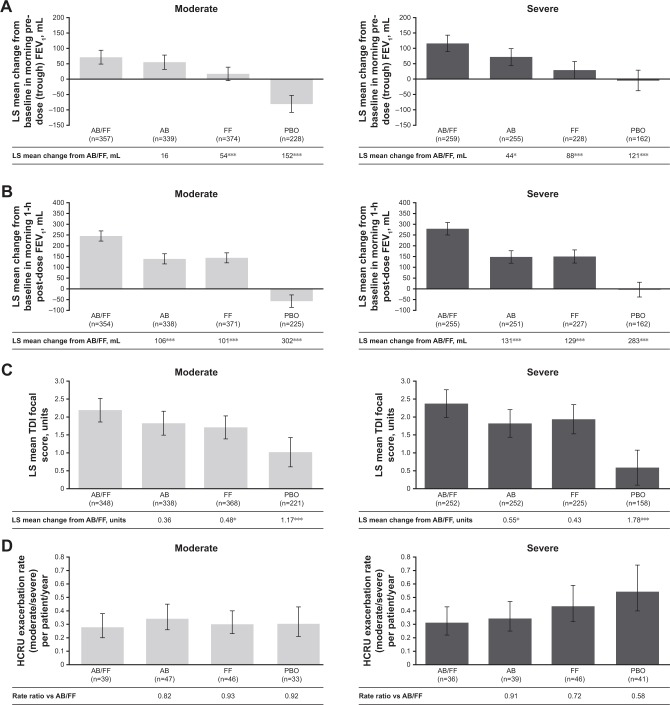
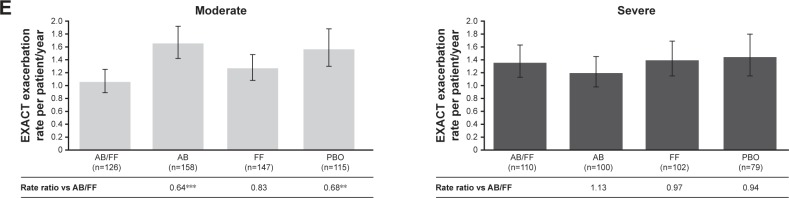
Figure 1.
Efficacy endpoints at week 24 analyzed by airflow obstruction severity (moderate vs severe).
Notes: (A) LS mean change from baseline in morning pre-dose (trough) FEV1;a (B) LS mean change from baseline in morning 1-hour post-dose FEV ;a 1 (C) LS mean TDI focal score;a (D) HCRU exacerbation rate (moderate/severe) per patient/year;b and (E) EXACT exacerbation rate per patient/yearb (pooled ITT population). *P<0.05; **P<0.01; and ***P<0.001. aAnalysis based on the mixed model for repeated measures. bAnalysis based on the negative binomial regression model. Error bars represent 95% CI.
Abbreviations: AB, aclidinium bromide; EXACT, EXAcerbations of Chronic pulmonary disease Tool; FF, formoterol fumarate; HCRU, healthcare resource utilization; ITT, intent-to-treat; LS, least squares; PBO, placebo; TDI, Transition Dyspnea Index.