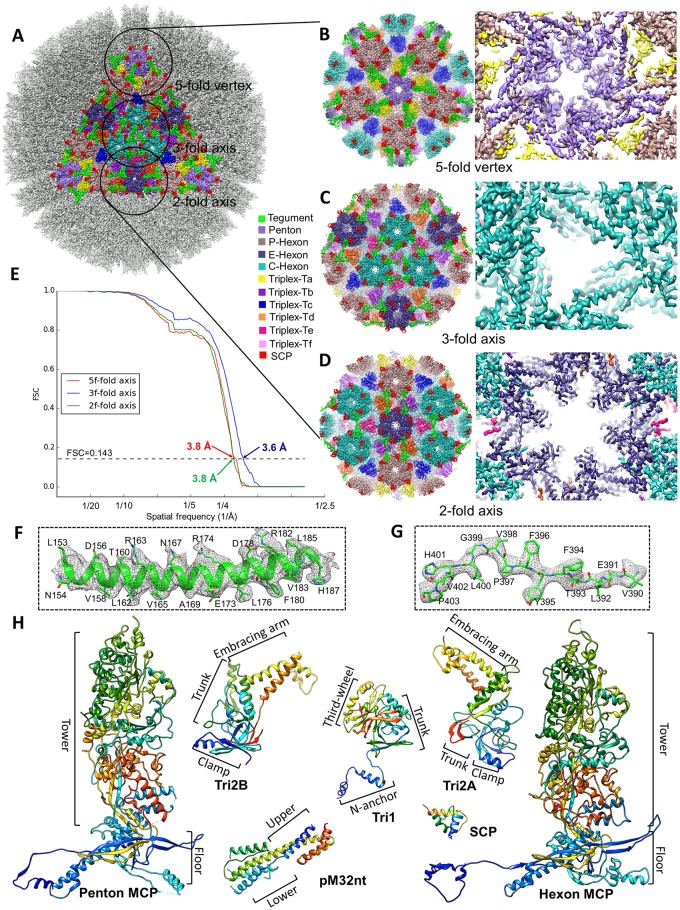
Fig 2. Resolution assessment, sub-particle reconstructions and MCMV atomic models.
(A-D) The icosahedral reconstruction in Fig 1A is reshown (A) with one triangular facet in color and regions (referred to as “sub-particles”) surrounding a 5-fold, 3-fold, and 2-fold axis circled. A total of 575,784 5-fold sub-particles, 959,640 3-fold sub-particles, and 1,439,460 2-fold sub-particles were boxed out from original particle images and refined to yield improved resolutions for the 5-fold sub-particle (B), 3-fold sub-particle (C), and 2-fold sub-particle (D). The enlarged views from inside (right panels) of these sub-particle reconstructions show α-helices and β-strands with well-resolved side chain densities. (E) Gold-standard (0.143) Fourier shell correlation (FSC) curves of the sub-particle reconstructions indicating the resolutions of sub-particle reconstructions at the 5-fold (red), 3-fold (blue), and 2-fold (green) axes are 3.8 Å, 3.6 Å, and 3.8 Å, respectively. (F-G) Close-up views of cryoEM density map (gray mesh) of an α-helix (F) and a loop (G) in the MCP floor region, superposed with atomic models (color). (H) De novo atomic models of individual capsid (MCP, SCP, Tri1, Tri2A, and Tri2B) and tegument (pM32nt) proteins shown as rainbow-colored ribbons (blue at the N-terminus to red at the C-terminus).