Figure 3: NHE1-3 loss-of-function affects neural tube closure as well as subapical actin formation and basal body positioning in MCCs.
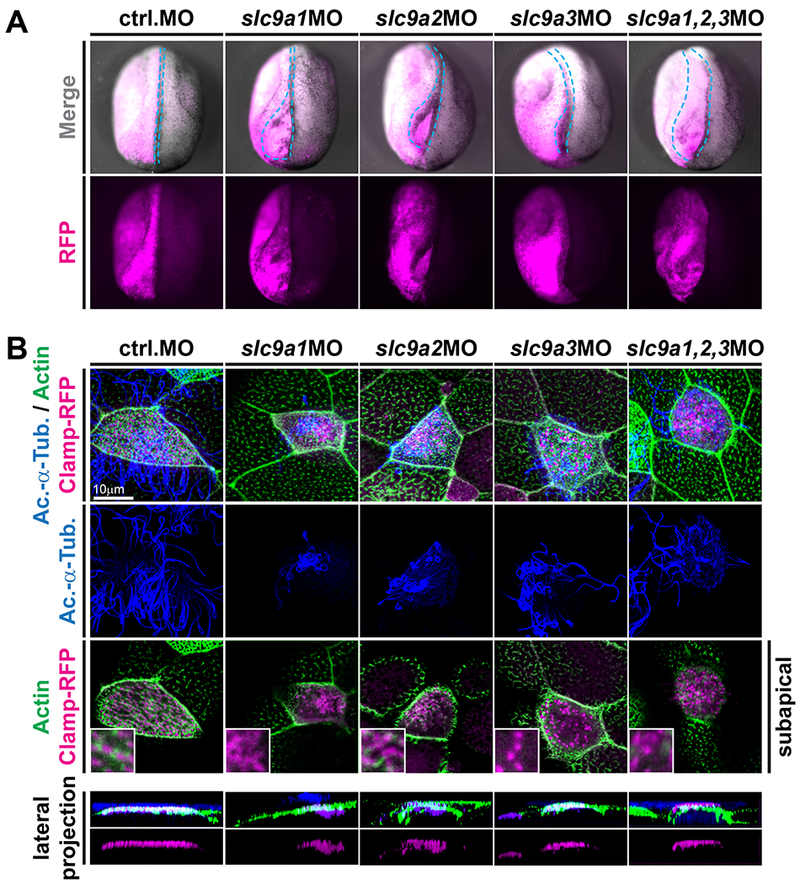
(A) slc9a1. 2 or 3 were knocked down unilaterally (visualized by co-injection of RFP encoding mRNA; individual knockdown at 4pmol, combined knockdown at 1.33pmol of each MO) and neural tube closure was analyzed at stage 19-20. In contrast to control-injected embryos and the non-injected control side of individual embryos, slc9a morphants displayed various degrees of neural tube closure defects (quantified in Fig. S2A). Representative examples are shown in anterior-dorsal view and the outline of the injected neural folds is marked by dashed blue line. (B) Embryos were injected with clamp-rfp (red) mRNAs and indicated MOs (same set of embryos as depiced in A). Immunofluorescence staining against acetylated-α-tubulin (Ac.-α-Tub., blue) revealed cilia and phalloidin stained the actin cytoskeleton (green). Loss of NHE function induced cilia defects as well as loss of subapical actin links between basal bodies in all treatments. Lateral projections further revealed severe basal body apical transport defects in slc9a1 and 2 morphants, but only mild or no defects after combined knockdown or after slc9a3MO injections. Scale bars indicate magnification.
