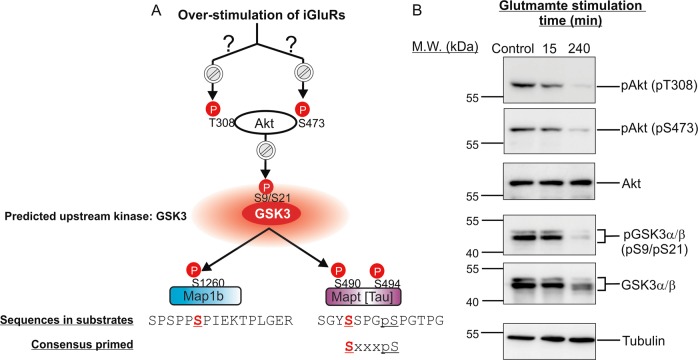
Fig. 7. Confirmation of the perturbation of GSK3 and Akt signalling activities in excitotoxicity predicted by changes of the phosphorylation states of Map1b and Mapt (Tau).
a A model depicting how glutamate overstimulation leads to inactivation of Akt and activation of GSK3 in neurons in excitotoxicity (top panel). GSK3 was previously found to phosphorylate Ser-1260 of Map1b and Ser-490 of Tau in vitro and in cells. GSK3 phosphorylation of Ser-490 of Tau is dependent on prior phosphorylation of Ser-494 by Cdk5. The sequences around Ser-1260 of Map1b and Ser-490 of Tau as well as the consensus phosphorylation-primed optimal sequences of GSK3 protein substrates are shown. The plots showing changes in phosphorylation states at Ser-1260 of Map1b and Ser-490/Ser-494 of Tau, presented as normalized log2 ratios of medium-versus light-labelled dimethyl derivatized tryptic phosphopeptides, are shown in Fig. 6. b Western blot analysis to follow the changes of phosphorylation states of the activating phosphorylation sites (Thr-308 and Ser-473) of Akt, and the inhibitory phosphorylation site (Ser-21 and Ser-9 of GSK3α and GSK3β) of the two GSK3 isoforms at 15 and 240 min after glutamate overstimulation. Besides the western blots probed with the phospho-specific antibodies, the western blots of total Akt, total GSK3α/β and tubulin are also presented