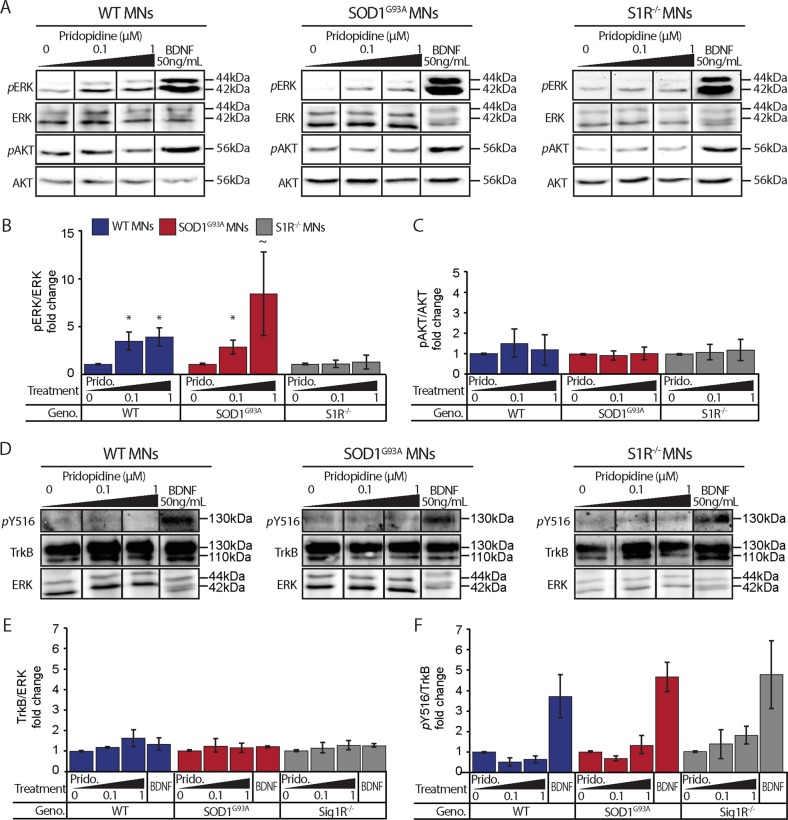
Fig. 5. Pridopidine increases ERK but not AKT and trkB phosphorylation in MNs.
a Western blots of WT, SOD1G93A, and S1R−/− MN culture extracts show an increase in the phosphorylation of ERK following a 30-minute incubation with 0.1 µM and 1 µM pridopidine in WT MNs (left panel) and SOD1G93A (middle panel), but not in S1R−/− MNs (right panel). AKT phosphorylation remained unaffected by pridopidine. Importantly, 50 ng/mL BDNF stimulation activates ERK and AKT in MNs from all sources. b Quantification of ERK reveals a ~3.5-4-fold increase in ERK phosphorylation in WT MNs following 0.1 µM and 1 µM pridopidine, respectively. SOD1G93A exhibited ~2.9-8.5-fold increase in ERK phosphorylation following 0.1 µM and 1 µM pridopidine, respectively. Pridopidine did not lead to ERK phosphorylation in S1R−/− MNs. c Quantification of AKT phosphorylation did not reveal any differences following pridopidine treatment. d Western blots of WT, SOD1G93A, and S1R−/− MN culture extracts show that the levels of TrkB and phospho(Thy516)TrkB in WT (left) SOD1G93A (center) and S1R−/− motor neurons do not change in response to either 0.1 µM or 1 µM pridopidine. BDNF 50 ng/mL was used as a positive control to demonstrate phospho(Thy516)TrkB activation in all MNs from all sources. e Quantification of TrkB levels reveals no change in its expression under all conditions. f Quantification of TrkB phosphorylation reveals that pridopidine does not activate theTrkB receptor at any of the tested concentrations. Data are shown as the mean pERK/ERK, pAKT/AKT, pTrkB/TrkB, or TrkB/Tubulin ratios ± SEM. *p value < 0.05, ~p value < 0.1 (n = 3 independent experiments; Student’s t test.)