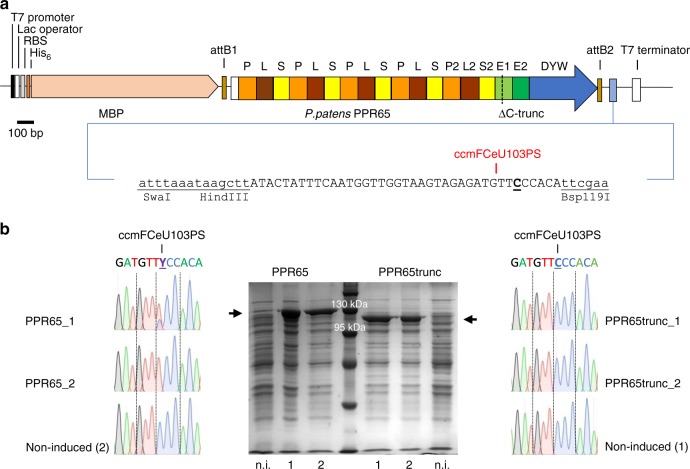
Fig. 1.
Strategy for establishing a plant C-to-U RNA editing setup in Escherichia coli, shown for P. patens pentatricopeptide repeat (PPR) protein PPR65 and its target editing site ccmFCeU103PS. a The PPR65 coding sequence is inserted into the pETG_41K vector system resulting in the fusion to a His6-tagged maltose binding protein (MBP, RBS: ribosome binding site). The editing target sequence is cloned downstream. Expression is driven by a T7 promoter inducible by isopropyl β-d-1-thiogalactopyranoside (IPTG). Editing site is labeled with target gene name (ccmFC encoding subunit FC of the cytochrome c maturation machinery) followed by eU, transcript position, and resulting amino acid change. b Editing of ccmFC103PS by PPR65 relies on its C-terminal domain in E. coli. Shown are protein expression and sequencing electropherograms revealing editing frequencies for two independent E. coli cultures with PPR65 and C terminally truncated PPR65, respectively, and non-induced samples as negative controls. Bacterial lysates on a denaturing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel correspond to ca. 4.5 × 107 cells of a 20-h culture after IPTG induction (n.i. = non-induced, PPR65trunc = C terminally truncated)