Abstract
Special high-K diets have cardioprotective effects and are often warranted in conjunction with diuretics such as furosemide for treating hypertension. However, it is not understood how a high-K diet (HK) influences the actions of diuretics on renal K+ handling. Furosemide acidifies the urine by increasing acid secretion via the Na+-H+ exchanger 3 (NHE3) in TAL and vacuolar H+-ATPase (V-ATPase) in the distal nephron. We previously found that an alkaline urine is required for large conductance Ca2+-activated K+ (BK)-αβ4-mediated K+ secretion in mice on HK. We therefore hypothesized that furosemide could reduce BK-αβ4-mediated K+ secretion by acidifying the urine. Treating with furosemide (drinking water) for 11 days led to decreased urine pH in both wild-type (WT) and BK-β4-knockout mice (BK-β4-KO) with increased V-ATPase expression and elevated plasma aldosterone levels. However, furosemide decreased renal K+ clearance and elevated plasma [K+] in WT but not BK-β4-KO. Western blotting and immunofluorescence staining showed that furosemide treatment decreased cortical expression of BK-β4 and reduced apical localization of BK-α in connecting tubules. Addition of the carbonic anhydrase inhibitor, acetazolamide, to furosemide water restored urine pH along with renal K+ clearance and plasma [K+] to control levels. Acetazolamide plus furosemide also restored the cortical expression of BK-β4 and BK-α in connecting tubules. These results indicate that in mice adapted to HK, furosemide reduces BK-αβ4-mediated K+ secretion by acidifying the urine.
Keywords: furosemide, high-K diet, large conductance Ca2+-activated K+, urine pH
INTRODUCTION
In contrast to the high-Na+, low-K+ Western diets, high-K+ diets such as the Mediterranean, Paleolithic, and vegetarian diets are well known for their protective benefits in cardiovascular and kidney diseases (25, 26, 28, 32, 63). These diets contain abundant fruits and vegetables and are naturally alkaline (13). As people become more aware of healthy eating habits (56), it is critical to understand how high-K+ diets influence the renal K+ handling to prevent drastic consequences of hyperkalemia.
Potassium is freely filtered into the glomerulus, reabsorbed in the proximal tubule and thick ascending limb (TAL), and secreted into the lumen of the distal nephron via the renal outer medullary K+ (ROMK) channel and the large conductance Ca2+-activated K+ (BK) channel. ROMK channels are considered constitutively active and secrete K+ at basal conditions (22, 36). By contrast, BK channels are activated by high urinary flow and secrete K+ under high-dietary K+ conditions (47, 64). In particular, the BK-αβ4 channel located in the intercalated cells of distal nephron (19) is required to maintain K+ homeostasis in mice on an alkaline high-K+ diet (8). The BK-β4 subunit of the BK-αβ4 channel is an accessory subunit that protects BK-α, the pore-forming subunit from lysosomal degradation (61). BK-β4 is regulated by the acidity/alkalinity of the diet (9). Compared with those on a high-KCl diet (HKCl), mice on an alkaline high-K+ diet (HK) exhibited increased BK-β4 expression and increased apical localization of BK-α (9). However, it is unclear whether BK-β4 is regulated by luminal or plasma pH.
Diuretics are among the most commonly used medications in the US. More than 20% of the US population over the age of 65 are taking diuretics (53). Numerous studies have shown the influences of high-dietary K+ intake on the actions and effectiveness of diuretics. Compared with those on a regular diet, mice on HK exhibit diminished thiazide-sensitive natriuresis due to decreased Na-Cl cotransporter (NCC) activity (49, 51) but enhanced amiloride-sensitive natriuresis due to upregulated epithelial Na+ channel (ENaC) activity (59, 60). Furosemide has reduced natriuretic effects on HK (50, 55) but increased efficacy on a low-Na+, high-K+ diet (55).
Additionally, furosemide is well known for its effect on acidifying the urine. Classically, this was thought to be due to increased Na+ delivery to distal nephron, generating a more favorable negative lumen potential for H+ secretion from the vacuolar H+-ATPase (V-ATPase) (16, 27). Chronic furosemide treatment increases V-ATPase expression (34). More recently, a study by de Bruijn et al. (11) showed that furosemide acidifies the urine by increasing H+ secretion via the Na+/H+ exchanger 3 (NHE3) in the TAL. Taking these together with the notion that BK-β4 is regulated by pH, we hypothesized that in mice on HK, furosemide reduces BK-αβ4-mediated K+ secretion by acidifying the urine.
METHODS
Animals.
All animals were maintained in accordance with and approved by the Institutional Animal Care and Use Committee of the University of Nebraska Medical Center (UNMC). Wild-type mice with C57BL/6 background (Charles River Laboratories, Wilmington, MA) and BK-β4-knockout (KO) mice with C57BL/6 background (generously provided by R. Brenner, Stanford University, Stanford, CA) were housed in the UNMC animal facility and maintained on a 12-h day-night cycle, with free access to water and food. Because no differences were observed between male and female mice, both sexes were used in this study.
Metabolic cage experiments.
Wild-type (WT) and BK-β4-knockout mice (BK-β4-KO), both male and female, at 12–16 wk of age, were kept on either a regular diet (RD; 0.3% Na, 0.6% K) or an alkaline high-K+ diet (HK; 5.0% K+ with 5% of equal carbonate-citrate-Cl and 0.32% Na+, TD.07278; Envigo, Indianapolis, IN). After drinking regular water for 7 days, they were then given either control water (0.7 mM KHCO3) or furosemide water (0.1 g/l furosemide + 0.7 mM KOH for HK, 0.3 g/l furosemide + 0.7 mM KOH for RD) for 11 days. Another group of WT and β4-KO mice were kept on HK for 7 days and then given furosemide plus acetazolamide water (0.1 g/l furosemide + 0.4 g/l acetazolamide + 2.0 mM KOH) for 7 days. All mice were acclimated to metabolic cages for 1 day each time for a total of three times before experiments. Urine was collected for 24 h in metabolic cages on the day before treatment water and 1, 4, 7, and 11 days after treatment. The mice were then anesthetized and euthanized by exsanguination from the carotid artery, and blood and kidneys were collected. Urine pH was measured with a pH meter (Model 215; Denver Instruments, Bohemia, NY). Arterial blood gas values and hematocrit were determined by the MN 300 i-STAT system with EC8+ cartridges (Abbott Point of Care, Princeton, NJ). Urine and plasma osmolality were measured with an osmometer (model 3250; Advanced Instruments, Norwood, MA). Urine and plasma Na+ and K+ concentrations were determined by a flame photometer (PFP7; Jenway, Burlington, NJ).
Immunofluorescence staining and quantification.
Kidneys were fixed with 2% paraformaldehyde as previously described (20). Mice were anesthetized with ketamine/xylazine cocktail, and chest cavity was opened. PBS containing 2% paraformaldehyde was perfused via the left ventricle for 5 min. The kidneys were then removed and cut into slices, which were fixed in 2% paraformaldehyde at 4°C overnight. Specimens were then embedded in paraffin and cut into serial sections on slides. Procedures for immunofluorescence staining were previously described (23). The following primary antibodies were used: anti-BK-α (mouse, diluted 1:100, UC Davis/NIH NeuroMab, Davis, CA), anti-V-ATPase B1 (rabbit, diluted 1:100, Santa Cruz), and anti-calbindin-D28K (mouse, diluted 1:100, Sigma, St. Louis, MO). The following secondary antibodies were applied for 1 h at room temperature: donkey anti-mouse IgG conjugated Alexa Fluor 488 and donkey anti-rabbit IgG conjugated Alexa Fluor 594 (diluted 1:200, Invitrogen, Carlsbad, CA). The stained sections were mounted using EMS Shield Mount with DABCO (Electron Microscopy Sciences, Hatfield, PA). Quantification of the BK-α signal intensity was determined following online instructions in single-channel, gray scale images after background correction as performed previously (23, 61). The apical ¼ as well as total cell intensity were measured for intercalated cells of the connecting tubules (CNT). For each group, kidney sections from three different animals were used, and at least three different tubules were analyzed per section.
Western blotting.
Kidneys were collected from euthanized mice, and kidney cortex was separated from medullary tissue and used for Western blotting, as previously described (10, 62). Kidney samples were placed in cold lysis buffer containing RIPA buffer + protease/phosphatase inhibitor (Sigma, St. Louis, MO) + 0.1% Triton X-100 (Sigma) + 1 mM PMSF (Sigma). Samples were cut into small pieces, sonicated, and kept on ice for 30 min before centrifuging at 12,500 g for 30 min at 4°C. Supernatant was collected, to which 4× Laemmli sample buffer (Bio-Rad, Hercules, CA) containing 10% β-mercaptoethanol (Sigma) was added. Samples were boiled for 5 min and loaded into 4–20% gradient gel (Bio-Rad) and run at 100 V for 1.5 h. The gel was then transferred onto Amersham Hybond-P membrane (GE Healthcare Life Sciences, Pittsburgh, PA) at 100 V for 1 h. The membrane was then blocked with 3% BSA, 3% nonfat milk in TBS-T for 1 h at room temperature. The membrane was then incubated with primary antibodies in blocking buffer overnight at 4°C. The next day, the blot was washed in TBS-T for 5 min three times and incubated with secondary antibodies for 1 h at room temperature. The blot was then washed and developed with Femto reagent (Thermo Fisher Scientific) and imaged. Primary antibodies included anti-BK-β4 (rabbit polyclonal, diluted 1:500; Alomone Laboratories, Jerusalem, Israel), anti-NHE3 (mouse monoclonal, diluted 1:200; Invitrogen, Carlsbad, CA), and anti-V-ATPase B1 (rabbit polyclonal, diluted 1:200; GeneTex, Irvine, CA). Secondary antibodies included goat anti-mouse IgG horseradish peroxidase (HRP)-conjugated (diluted 1:15,000; Santa Cruz Biotechnology, Santa Cruz, CA), goat anti-rabbit IgG HRP-conjugated (diluted 1:15,000, Santa Cruz Biotechnology, Santa Cruz, CA), and anti-β-actin HRP-conjugated (diluted 1:15,000; Thermo Fisher Scientific, Waltham, MA). Densitometry analysis was performed using ImageJ software.
Measurements of aldosterone and furosemide.
Plasma aldosterone levels were measured as previously described (10) using the aldosterone ELISA kit EIA-5298 (DRG Diagnostics, Marburg, Germany), following manufacturer’s protocol.
The concentrations of furosemide in urine were determined with high-performance liquid chromatography (HPLC), as previously described (40). The instrument (Agilent LC 1200; Agilent, Santa Clara, CA) was connected to a G1321A Agilent fluorescent detector, and the chromatograms were recorded using Agilent ChemStation software. A C18 column (10.0 µm; Waters Spherisorb) was used as the stationary phase. The mobile phase consisted of cetonitrile-0.125 M SDS-0.01 M perchloric acid (234.6:35:665, wt/wt) pumped at a constant flow rate of 0.6 ml/min. The retention time of furosemide was 3.7 min and was separated from the rest of the compounds present in urine and plasma in chromatographs obtained with excitation at 360 nm and emission at 413 nm. A standard curve was constructed using the areas under the furosemide peak of plasma samples containing 0, 10, 20, 50, 100, 200, 500, 1,000, 2,000, and 5000 µM furosemide. The furosemide concentrations of urine samples were then calculated using the standard curve.
Statistical analyses.
Data shown in figures and tables represent means ± SE. Significant differences between each group were determined by Student’s t-test, two-way ANOVA with post-hoc Tukey test, or two-way repeated measures ANOVA with post hoc Holm-Sidak test (P < 0.05 considered significant), as indicated.
RESULTS
Furosemide effect on urine pH.
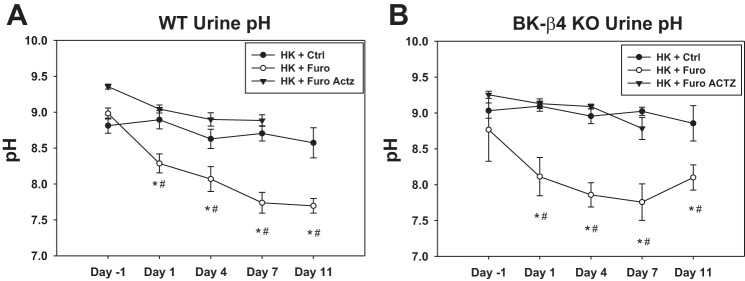
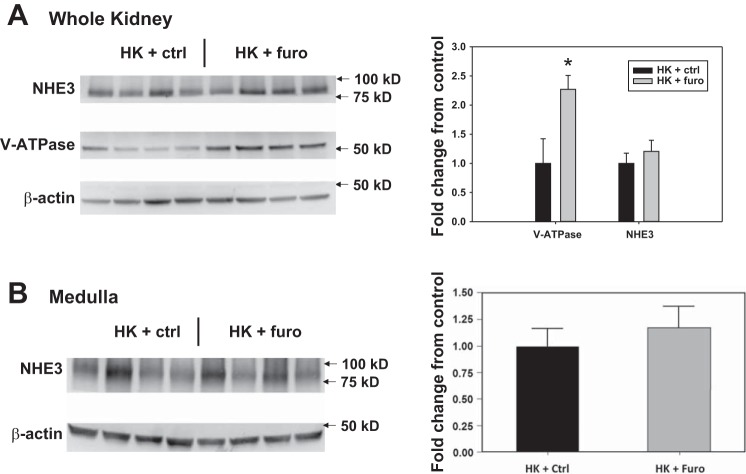
Consistent with the urine acidification effect of furosemide (2, 11, 24, 54), our results showed that over the course of treatment, furosemide water (Furo) reduced urine pH in both WT and β4-KO on HK (Fig. 1). However, blood pH was not different between the HK + Ctrl and HK + Furo group (Table 1). Notably, urine pH kept decreasing until day 7. This gradual reduction could be due to increased V-ATPase expression in the distal nephron (34). Indeed, compared with the HK + Ctrl group, the HK + Furo group had higher V-ATPase expression (Fig. 2A). The NHE3 expression remained unchanged in the whole kidney and the medulla (Fig. 2). Because V-ATPase expression is regulated by aldosterone, we measured the plasma aldosterone levels. The HK + Furo group had significantly higher plasma aldosterone levels compared with HK + Ctrl group (Table 1).
Fig. 1.
Furosemide (Furo) effect on urine pH. Urine pH of wild-type (WT; A) and β4-knockout (β4-KO; B) on day −1, 1, 4, 7, and 11 of control (Ctrl) and Furo treatments or on day −1, 1, 4, and 7 of Furo + acetazolamide (Actz) treatment. *P < 0.05 vs. HK + Ctrl water, #P < 0.05 vs. Day –1, analyzed with two-way repeated-measures ANOVA with a post hoc Holm-Sidak test (P < 0.001 for both treatment and days with interaction P < 0.01). n = 12–14 for WT Ctrl and Furo; N = 7 – 8 for β4-KO Ctrl and Furo; N = 4 for WT Furo + Actz; n = 3 for β4-KO Furo + Actz.
Table 1.
Blood measurements after 11 days (or 7 days for HK + Furo Actz) of treatment
| Genotype |
|||||||
|---|---|---|---|---|---|---|---|
| WT |
BK-β4-KO |
||||||
| Treatment | RD + Ctrl | RD + Furo | HK + Ctrl | HK + Furo | HK + Furo Actz | HK + Ctrl | HK + Furo |
| No. of mice | 4 | 6 | 12 | 14 | 4 | 8 | 7 |
| P [Na+], mM | 139.8 ± 1.2 | 141.9 ± 1.6 | 137.6 ± 1.1 | 140.9 ± 1.0* | 144.3 ± 5.0 | 139.8 ± 1.2 | 140.1 ± 1.0 |
| Hct, % | 37.0 ± 0.6 | 41.7 ± 0.9* | 36.5 ± 0.3 | 39.8 ± 0.5* | 35.5 ± 1.0† | 35.4 ± 0.3 | 38.0 ± 1.2* |
| Blood pH | 7.46 ± 0.02 | 7.51 ± 0.05 | 7.46 ± 0.02 | 7.45 ± 0.03 | 7.45 ± 0.02 | 7.42 ± 0.04 | 7.45 ± 0.03 |
| P Aldo, pg/ml | 862 ± 165 (n = 7) | 1,489 ± 179 (n = 8)* | |||||
Values are means ± SE. Actz, acetazolamide; BK-β4-KO, large conductance Ca2+-activated K+-β4-knockout; Ctrl, control; Furo, furosemide; Hct, hematocrit; HK, high-K diet; P Aldo, plasma aldosterone; RD, regular diet; WT, wild type.
P < 0.05 vs. Ctrl group;
P < 0.05 vs. Furo group analyzed with two-way ANOVA with a post hoc Tukey test.
Fig. 2.
Western blot and quantification of Na+/H+ exchanger 3 (NHE3) and vacuolar H+-ATPase (V-ATPase) expression of whole kidney (A) and medulla (B) of mice treated with HK + control (Ctrl) and HK + furosemide (Furo). *P < 0.05 vs. HK + Ctrl analyzed with Student’s t-test; n = 4/group.
Furosemide effect on renal K+ handling.
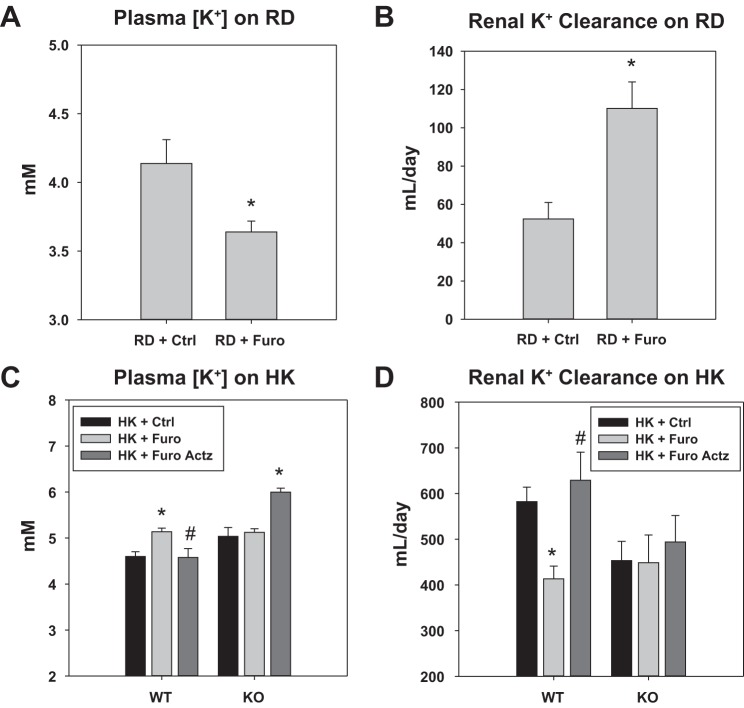
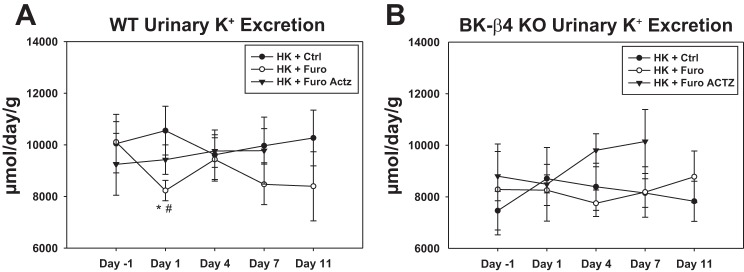
As a well-known K+-wasting diuretic, in mice on RD, the Furo group had a significantly lower plasma [K+] and higher K+ clearance compared with the Ctrl water group (Fig. 4, A and B). However, in WT on HK, Furo treatment reduced urinary K+ excretion (UKV̇) on day 1 (Fig. 3). After 11 days of treatment, the HK + Furo group had a lower K+ clearance and higher plasma [K+] than the HK + Ctrl group (Fig. 4, C and D). The food intake was similar between the two groups throughout the treatment period. This indicates that furosemide becomes a K-sparing diuretic in mice on HK. Additionally, this effect is dependent on BK-β4 since furosemide did not affect K+ clearance or plasma [K+] in β4-KO on HK (Fig. 4, C and D).
Fig. 4.
Furosemide (Furo) effect on plasma [K+] and renal K+ clearance in mice on regular diet (RD) and high-K+ diet (HK). A and B: plasma [K+] and renal K+ clearance of WT on RD on day 11 of control (Ctrl) or Furo treatments. *P < 0.05 vs. RD + Ctrl analyzed with Student’s t-test; n = 4 for Ctrl, n = 6 for Furo. C and D: plasma [K+] and renal K+ clearance of WT and β4-knockout (β4-KO) on day 11 of Ctrl and Furo treatment or day 7 of Furo + acetazolamide (Actz) treatment. *P < 0.05 vs. HK + Ctrl; #P < 0.05 vs. HK + Furo, analyzed with two-way ANOVA with a post hoc Tukey test (P < 0.001 for treatment in plasma [K+]; P < 0.01 for treatment in renal K+ clearance); n = 12 – 14 for WT HK + Ctrl and HK + Furo; n = 7 – 8 for β4-KO HK + Ctrl and HK + Furo; n = 4 for WT HK + Furo Actz; n = 3 for β4-KO HK + Furo Actz.
Fig. 3.
Furosemide (Furo) effect on urinary K+ excretion (UKV̇) normalized to kidney weight in mice on high-K diet (HK). UKV̇ of wild-type (WT; A) and β4-knockout (β4-KO; B) on day −1, 1, 4, 7, and 11 of control (Ctrl) and Furo treatment or on day −1, 1, 4, and 7 of Furo + acetazolamide (Actz) treatment. *P < 0.05 vs. HK + Ctrl; #P < 0.05 vs. day −1, analyzed with two-way repeated measures ANOVA (P < 0.05 for drug treatment in WT) with a post hoc Holm-Sidak test; n = 12–14 for WT HK + Ctrl and HK + Furo; n = 7–8 for β4-KO HK + Ctrl and HK + Furo; n = 4 for WT HK + Furo Actz; n = 3 for β4-KO HK + Furo Actz.
To test whether the furosemide effect on K+ excretion is due to urine acidification, another group of mice on HK were treated with furosemide water with acetazolamide added to alkalinize the urine (HK + Furo Actz). Because of volume depletion in HK + Furo Actz mice, the treatment was 7 days instead of 11 days. The addition of Actz successfully kept the urine alkaline without affecting the blood pH (Fig. 1 and Table 1). In WT, unlike HK + Furo, the HK + Furo Actz group showed similar UKV̇, plasma [K+] and renal K+ clearance to the HK + Ctrl group (Fig. 4, C and D). In β4-KO mice, the HK + Furo Actz group showed similar UKV̇ and renal K+ clearance but higher plasma [K+] compared with the HK + Ctrl group (Fig. 4, C and D) The combination of furosemide and acetazolamide produced a more profound diuresis than furosemide alone (Table 2), consistent with the recognized synergistic effect of these two diuretics (5, 6).
Table 2.
Metabolic cage measurements after 11 days (or 7 days for HK + Furo Actz) of treatment
| Genotype |
||||||||
|---|---|---|---|---|---|---|---|---|
| WT |
BK-β4-KO |
|||||||
| Treatment | RD + Ctrl | RD + Furo | HK + Ctrl | HK + Furo | HK + Furo Actz | HK + Ctrl | HK + Furo | HK + Furo Actz |
| No. of mice | 4 | 6 | 12 | 14 | 4 | 8 | 7 | 3 |
| ΔBW, g | −0.4 ± 0.1 | −2.2 ± 0.3* | 0.9 ± 0.5 | −0.6 ± 0.5* | −2.9 ± 0.7*† | 1.0 ± 0.6 | −1.1 ± 0.5* | −2.6 ± 0.6*† |
| V̇, ml/day | 0.7 ± 0.2 | 2.6 ± 0.7 | 7.2 ± 0.7 | 7.1 ± 0.6 | 11.6 ± 1.7*† | 5.4 ± 0.6 | 6.8 ± 0.7 | 11.6 ± 1.4*† |
| U Osm, mosmol/kgH2O | 2,725 ± 452 | 1,367 ± 451* | 1,020 ± 73 | 809 ± 47* | 696 ± 38* | 1173 ± 42 | 908 ± 81* | 1,060 ± 67 |
Values are means ± SE. Actz, acetazolamide; BK-β4-KO, large conductance Ca2+-activated K+-β4-knockout; BW, body weight; Ctrl, control; Furo, furosemide; HK, high-K diet; U Osm, urinary osmolality; RD, regular diet; V̇, urine flow; WT, wild type.
P < 0.05 vs. Ctrl group;
P < 0.05 vs. Furo group analyzed with two-way ANOVA with a post hoc Tukey test.
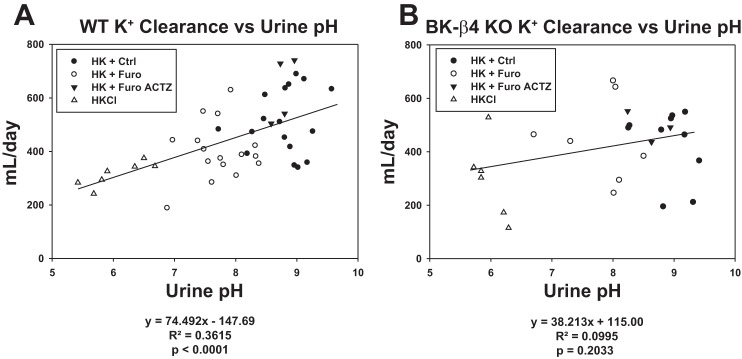
Luminal pH is well known to be positively correlated with K+ secretion in the distal nephron (31, 33). To illustrate this relationship, we plotted renal K+ clearance versus urine pH and included data from WT and β4-KO mice maintained on HKCl diet. Consistent with this notion, in WT on HK, renal K+ clearance was positively correlated with urine pH (Fig. 5A). However, such a correlation was not significant for β4-KO on HK (Fig. 5B). This indicates that the correlation between urine pH and K+ clearance was dependent on BK-β4 expression.
Fig. 5.
Correlation between renal K+ clearance and urine pH in wild-type (WT; A) and β4-knockout (β4-KO; B) on high-K+ diet (HK). ●, HK + control (Ctrl) group; ○, HK + furosemide (Furo) group; ▲, HK + Furo acetazolamide (Actz) group; △, HKCl group.
Furosemide effect on BK-αβ4 expression.
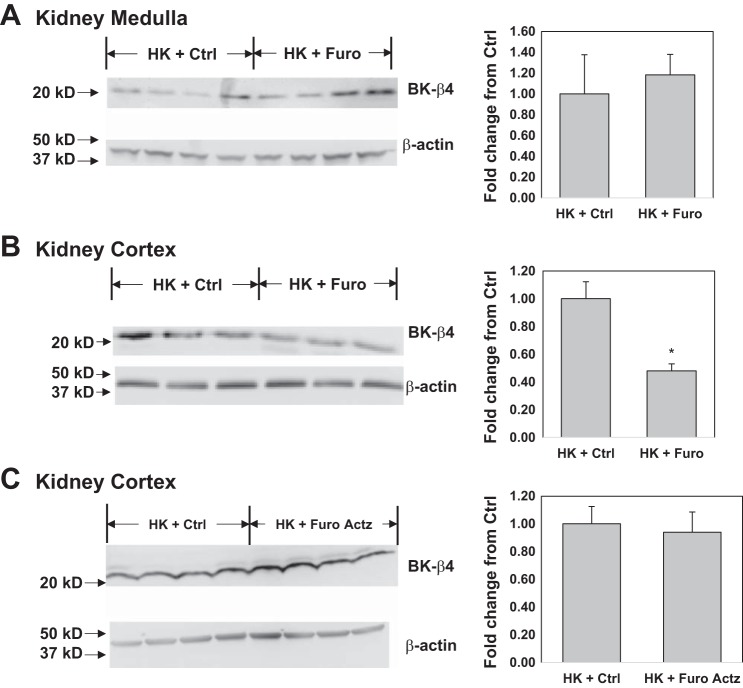
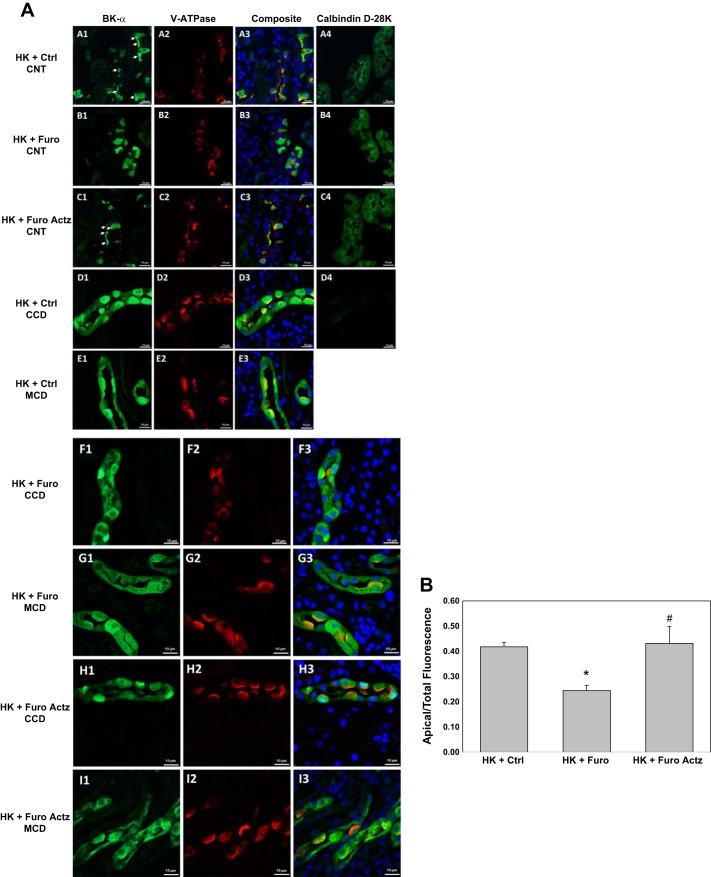
Previous studies showed that the alkalinity/acidity of diets regulates the BK-αβ4-mediated K+ secretion by altering BK-β4 expression, which is required for maintaining the apical localization of BK-α (9, 61). We hypothesized that furosemide would have similar effects by acidifying the urine. Shown by the Western blot in Fig. 6 and the immunofluorescent staining in Fig. 7, compared with the HK + Ctrl group, HK + Furo but not HK + Furo Actz had significantly reduced BK-β4 expression and decreased apical localization of BK-α in the kidney cortex. In the HK + Ctrl group, BK-α was located in the apical region of ICs of CNT but not cortical collecting duct (CCD) or medullary collecting duct (MCD), as evidenced by costaining with V-ATPase (IC marker) and calbindin [distal convoluted tubule (DCT)/CNT marker]. Consistent with this differential regulation of BK-α in different nephron segments, the BK-β4 expression in the kidney medulla remained unaffected by Furo treatment (Fig. 6A).
Fig. 6.
Furosemide (Furo) effect on large conductance Ca2+-activated K+ (BK)-β4 expression in the kidney. A: BK-β4 expression in the kidney medulla of wild-type (WT) on high-K diet (HK) treated with control (Ctrl) or Furo water; n = 4/group. B: BK-β4 expression in the kidney cortex of WT on HK treated with Ctrl vs. Furo water. *P < 0.05 vs. HK + Ctrl analyzed with Student’s t-test; n = 7/group. C: BK-β4 expression in the kidney cortex of WT on HK treated with Ctrl vs. Furo acetazolamide (Actz) water; n = 4/group.
Fig. 7.
Large conductance Ca2+-activated K+ (BK)-α localization in kidney sections of wild-type (WT) mice on high-K diet (HK) treated with control (Ctrl), furosemide (Furo), or Furo + acetazolamide (Actz) water. A: representative images from the connecting tubules (CNT) of HK + Ctrl (images A1, A2, A3, and A4), CNT of HK + Furo (images B1, B2, B3, and B4), CNT of HK + Furo Actz (images C1, C2, C3, and C4), CCD of HK + Ctrl (images D1, D2, D3, and D4), medullary collecting duct (MCD) of HK + Ctrl (images E1, E2, and E3), cortical collecting duct (CCD) of HK + Furo (images F1, F2, and F3), MCD of HK + Furo (images G1, G2, and G3), CCD of HK + Furo Actz (images H1, H2, and H3), and MCD of HK + Furo Actz (images I1, I2, and I3). Images A1, B1, C1, D1, E1, F1, G1, H1, and I1 were stained with anti-BK-α in green; images A2, B2, C2, D2, E2, F2, G2, H2, and I2 were stained with anti-V-ATPase in red; A3, B3, C3, D3, E3, F3, G3, H3, and I3 are composite images of all those in the first two columns, with nucleus staining DAPI in blue; images A4, B4, C4, and D4 are serial sections of their respective rows of images and were stained with anti-calbidin-D28K in green. Scale bars, 10 µm. B: ratio of BK-α intensity in the apical ¼ to the whole intercalated cells. *P < 0.001 vs. HK + Ctrl; #P < 0.01 vs. HK + Furo analyzed with Student’s t-test.
DISCUSSION
The results of the present study demonstrated the following: 1) chronic furosemide treatment acidified the urine with increased V-ATPase expression; 2) chronic furosemide treatment reduced renal K+ clearance and increased plasma [K+] in WT but not BK-β4 KO on HK; 3) chronic furosemide treatment downregulated BK-β4 expression in the cortex and decreased BK-α localization to the apical region of the intercalated cells of CNT in WT on HK; 4) the effect of furosemide on K+ handling and BK-αβ4 was prevented by alkalinizing the urine with acetazolamide.
Furosemide is a commonly used K-wasting diuretic, and patients taking furosemide often require K+ supplements to prevent hypokalemia. However, our current study found that furosemide became a K-sparing diuretic in mice adapted to HK. Instead of a risk for hypokalemia, furosemide increased the plasma [K+] in these mice. In one study, furosemide and acetazolamide separately increased K+ excretion in rats; however, when furosemide was added during acetazolamide administration, K+ excretion was reduced despite profoundly increased Na+ excretion (45). This phenomenon can be explained by our findings that urine pH regulates BK-αβ4-mediated K+ secretion. During acetazolamide administration, urine was alkalinized, increasing BK-αβ4-mediated K+ secretion, contributing to the kaliuretic effect of acetazolamide. When furosemide was added, urine was acidified, reducing BK-αβ4-mediated K+ secretion, leading to decreased kaliuresis despite increased natriuresis. In a clinical study, when furosemide and thiazide, an inhibitor of the sodium chloride cotransporter (NCC) in the distal tubule, were administered in doses with equal natriuretic effects, the kaliuresis induced by furosemide was lower than that of hydrochlorothiazide (42). This phenomenon has been explained by the fact that during furosemide treatment, the unabsorbed Na+ in the TAL was extracted by the NCC in the early DCT, reducing the delivery of Na+ to distal K+ secretory sites (42, 43). However, our study provides an alternative explanation, i.e., that furosemide inhibits BK-mediated K+ secretion, leading to a lower kaliuretic effect than thiazide.
Urinary acidification by furosemide.
Furosemide causes marked urine acidification and is used to diagnose distal renal tubular acidosis (2, 39, 48). The classical explanation was similar to the K-wasting effect of furosemide. The increased distal Na+ delivery to ENaC generates a more negative lumen potential in the ASDN for both K+ and H+ secretion (16). The study by Kovacikova et al. (27) also showed that the acute urine acidification was dependent on ENaC in the CNT. However, the recent study by de Bruijn et al. (11) showed that acutely furosemide-induced urine acidification was caused by increased H+ secretion from NHE3 in the TAL. Another study showed that chronic furosemide treatment in mice on a regular diet acidified urine in an ENaC-independent manner and increased V-ATPase expression without changes in plasma aldosterone (34).
Consistent with previous studies, our data showed that, chronically, furosemide reduced the urine pH starting from the 1st day of treatment and caused a gradual decrease until day 7. After 11 days of treatment, the V-ATPase expression in the whole kidney was doubled. However, unlike the previous study (34), our data showed increased plasma aldosterone levels in the HK + Furo group, which is consistent with their elevated plasma [K+]. Furthermore, by inhibiting Na+ reabsorption in TAL, furosemide inhibits Ca2+ reabsorption in the TAL and sends more Na+ to be reabsorbed by NCC and ENaC. Increased ENaC-mediated Na reabsorption reduces the driving force for Na+-Ca2+ exchange by Na+/Ca2+ exchanger 1 (NCX1), inhibiting Ca2+ reabsorption in the DCT and CNT (15, 29). In addition to aldosterone, the activity of V-ATPase may be stimulated by the increased urinary [Ca2+] via the action of the Ca-sensing receptor (CaSR) (41). Although the NHE3 expression remained unchanged by furosemide, the synergistic effect of furosemide plus acetazolamide suggests that NHE3 activity may be upregulated during furosemide treatment and contribute to the urine acidification by furosemide.
Furosemide and BK-αβ4-mediated K+ secretion.
The link between pH and BK-αβ4 has been previously demonstrated by our lab. As the diet becomes more acidic, BK-β4 expression is decreased, reducing apical localization of BK-α (9, 10, 61). In the current study, chronic furosemide treatment reduced urine pH, BK-β4 expression and apical localization of BK-α in WT on HK. This effect remarkably overrides the K-wasting effect from increased distal Na+ delivery, resulting in an overall decrease in renal K+ clearance and an elevation of plasma [K+]. In fact, furosemide did not cause a significant increase in urine flow (V̇) or UNaV̇ in mice on HK despite the fact that the urinary concentration of furosemide measured with HPLC was 683 ± 97 µM, ∼50-fold higher than its IC50 (7.2–15.1 µM) (Table 3) (7). This could be because NKCC2 activity is partly inhibited in mice on HK (50, 55). However, the effectiveness of furosemide was still demonstrated by the lower urine osmolality, reduced body weight, higher hematocrit, higher plasma aldosterone, and reduced urine pH in HK + Furo compared with the HK + Ctrl group. Moreover, the less profound diuretic and natriuretic effect of furosemide under HK conditions did not stimulate ROMK and BK-αβ1-mediated K+ secretion as much as regular dietary conditions. This might have caused the reduction in BK-αβ4-mediated K+ secretion to be the predominant effect and resulted in a net decrease in K+ excretion.
Table 3.
Furosemide concentrations in urine and plasma of WT and BK-β4-KO
| WT HK + Furo | BK-β4-KO HK + Furo | |
|---|---|---|
| No. of animals | 4 | 5 |
| [Furo] in urine, µM | 683 ± 97 | 610 ± 123 |
| [Furo] in plasma, µM | 6.5 ± 3.2 | 5.6 ± 2.0 |
Values are means ± SE. BK-β4-KO, large conductance Ca2+-activated K+-β4-knockout; Furo, furosemide; HK, high-K diet; WT, wild type.
Furosemide acidified the urine of BK-β4-KO mice on HK without affecting renal K+ clearance or plasma [K+], which were comparable to those of WT HK + Furo. These results suggest that the K-sparing effect of furosemide in WT on HK is due to the inhibition of BK-αβ4-mediated K+ secretion. The K-sparing effect was abolished when the urine was alkalinized with acetazolamide. This could also be due to the further increase in distal Na+ delivery stimulating K+ secretion. However, this is unlikely given that BK-β4 expression and BK-α localization were similar between HK + Furo and HK + Furo Actz groups in WT. Furthermore, despite increased natriuresis, HK + Furo Actz did not have a higher renal K+ clearance than the HK + Furo group in BK-β4-KO.
Another finding is that pH regulation of BK-αβ4 occurs in the CNT but not CCD or MCD. This was supported by both IF staining, where BK-α was localized in the apical region of CNT only, and the Western blot, where BK-β4 expression was only reduced in the kidney cortex. CNT has been identified as the major site of K+ secretion (27) and furosemide-induced H+ secretion (27, 46). According to our IF staining, BK-α was almost exclusively expressed in the ICs of the CNT. This agrees with the notion that ICs play a major role in BK-mediated K+ secretion (35, 58).
Mechanism of alkalinity-induced BK-αβ4-mediated K secretion.
The association between luminal pH and K+ secretion is well documented (1, 31, 33). Luminal acidification from pH 7.4 to 6.8 impairs K+ secretion in the rabbit CCD without changing Na+ reabsorption (4). Although ROMK channels are regulated by cytosolic pH (14, 33), they are insensitive to extracellular pH (14, 17). The intracellular pH of PCs is unlikely to be affected by luminal pH due to the lack of acid base transporters in the apical membrane (57).
The link between luminal pH and K+ secretion has been attributed to several possibilities, including a pH-sensitive apical K-Cl cotransporter in PCs (1), a TASK2 K+ channel that is regulated by extracellular pH (1), and ENaC modulation by luminal (37). Our current study provides another explanation that luminal pH regulates BK-αβ4-mediated K+ secretion, as evidenced by our finding that BK-β4 is required for the positive correlation between urine pH and renal K+ clearance.
The mechanism by which luminal pH regulates BK-β4 expression remains unclear. The pH needs to be sensed from the luminal side of ICs. The CaSR, located in the apical membrane of both PCs and ICs of the DCT and CD (44), is a possible pH sensor. Extracellular alkalinization above pH 6 increases the sensitivity of CaSR to extracellular Ca2+ (12, 38). The activation of CaSR would release Ca2+ from intracellular stores and increase Ca2+ entry via TRPV5 channels (52) to further activate BK channels (21). The TRPV4 channel plays a critical role in upregulating BK-mediated K+ secretion during adaptation to HK (3). The coupling of CaSR and TRPV4 was recently demonstrated in gastric cancer cells (65); however, such a relationship in the kidney remains to be investigated.
Limitations and conclusions.
One limitation of our study is the use of drinking water for drug treatment. This method is noninvasive and minimizes stress but also makes drug dosage different in each animal. Although the water intake is not statistically different among the different groups on HK, it may vary among individual mice, leading to larger standard errors. Additionally, KOH was added in drinking water to dissolve furosemide and acetazolamide, resulting in unequal K+ intake from water. However, because K+ intake from water (20 µmol/day) is <1% of the daily urinary K+ output (∼2,500 µmol/day), it is unlikely to affect our results. Another limitation of our study is that high doses of furosemide may inhibit NCC in the DCT. However, NCC activity is already strongly inhibited in mice on HK due to elevated plasma [K+] (51, 59). Furosemide should not have a significant effect on NCC in these mice.
Another explanation for the K-sparing effect of furosemide is that it may inhibit NKCC1 in the basolateral membrane of ICs to limit BK-mediated K+ secretion (30). However, the plasma concentrations of furosemide (Table 3) in the HK + Furo group (WT: 6.5 ± 3.2 µM; BK-β4-KO: 5.6 ± 2.0 µM) were much lower than its IC50 (23 ± 12 µM) (18). Additionally, the restoration of BK-β4 expression and BK-α localization by coadministration of acetazolamide strongly supports the notion that furosemide exerts its effect on BK-αβ4 via urinary acidification.
In conclusion, our study indicates that furosemide decreases BK-αβ4-mediated K+ secretion by acidifying the urine, making furosemide a K-sparing diuretic in mice adapted to an alkaline high-K+ diet.
GRANTS
This project was funded by National Institute of Diabetes and Digestive and Kidney Diseases Grants RO1 DK071014 (S. C. Sansom), RO1 DK92474 (S. C. Sansom), and F30 DK108456 (B. Wang).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.W. conceived and designed research; B.W., J.W.-F., and H.L. performed experiments; B.W., J.W.-F., and H.L. analyzed data; B.W., J.W.-F., H.L., and S.C.S. interpreted results of experiments; B.W. prepared figures; B.W. drafted manuscript; B.W., J.W.-F., and S.C.S. edited and revised manuscript; B.W. and S.C.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We appreciate receiving the BK-β4-knockout mice from Dr. Robert Brenner (Stanford University, Stanford, CA) and the BK-α-knockout mouse kidney tissue from Dr. Andrea Meredith and Dr. Paul Welling (University of Maryland School of Medicine, Baltimore, MD).
REFERENCES
- 1.Amorim JB, Bailey MA, Musa-Aziz R, Giebisch G, Malnic G. Role of luminal anion and pH in distal tubule potassium secretion. Am J Physiol Renal Physiol 284: F381–F388, 2003. doi: 10.1152/ajprenal.00236.2002. [DOI] [PubMed] [Google Scholar]
- 2.Batlle DC. Segmental characterization of defects in collecting tubule acidification. Kidney Int 30: 546–554, 1986. doi: 10.1038/ki.1986.220. [DOI] [PubMed] [Google Scholar]
- 3.Berrout J, Jin M, Mamenko M, Zaika O, Pochynyuk O, O’Neil RG. Function of transient receptor potential cation channel subfamily V member 4 (TRPV4) as a mechanical transducer in flow-sensitive segments of renal collecting duct system. J Biol Chem 287: 8782–8791, 2012. doi: 10.1074/jbc.M111.308411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boudry JF, Stoner LC, Burg MB. Effect of acid lumen pH on potassium transport in renal cortical collecting tubules. Am J Physiol 230: 239–244, 1976. [DOI] [PubMed] [Google Scholar]
- 5.Brater DC. Diuretic therapy. N Engl J Med 339: 387–395, 1998. doi: 10.1056/NEJM199808063390607. [DOI] [PubMed] [Google Scholar]
- 6.Brater DC, Kaojarern S, Chennavasin P. Pharmacodynamics of the diuretic effects of aminophylline and acetazolamide alone and combined with furosemide in normal subjects. J Pharmacol Exp Ther 227: 92–97, 1983. [PubMed] [Google Scholar]
- 7.Carota I, Theilig F, Oppermann M, Kongsuphol P, Rosenauer A, Schreiber R, Jensen BL, Walter S, Kunzelmann K, Castrop H. Localization and functional characterization of the human NKCC2 isoforms. Acta Physiol (Oxf) 199: 327–338, 2010 10.1111/j.1748-1716.2010.02099.x. [DOI] [PubMed] [Google Scholar]
- 8.Cornelius RJ, Wang B, Wang-France J, Sansom SC. Maintaining K+ balance on the low-Na+, high-K+ diet. Am J Physiol Renal Physiol 310: F581–F595, 2016. doi: 10.1152/ajprenal.00330.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cornelius RJ, Wen D, Hatcher LI, Sansom SC. Bicarbonate promotes BK-α/β4-mediated K excretion in the renal distal nephron. Am J Physiol Renal Physiol 303: F1563–F1571, 2012. doi: 10.1152/ajprenal.00490.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cornelius RJ, Wen D, Li H, Yuan Y, Wang-France J, Warner PC, Sansom SC. Low Na, high K diet and the role of aldosterone in BK-mediated K excretion. PLoS One 10: e0115515, 2015. doi: 10.1371/journal.pone.0115515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Bruijn PI, Larsen CK, Frische S, Himmerkus N, Praetorius HA, Bleich M, Leipziger J. Furosemide-induced urinary acidification is caused by pronounced H+ secretion in the thick ascending limb. Am J Physiol Renal Physiol 309: F146–F153, 2015. doi: 10.1152/ajprenal.00154.2015. [DOI] [PubMed] [Google Scholar]
- 12.Doroszewicz J, Waldegger P, Jeck N, Seyberth H, Waldegger S. pH dependence of extracellular calcium sensing receptor activity determined by a novel technique. Kidney Int 67: 187–192, 2005. doi: 10.1111/j.1523-1755.2005.00069.x. [DOI] [PubMed] [Google Scholar]
- 13.Eaton SB, Konner M. Paleolithic nutrition. A consideration of its nature and current implications. N Engl J Med 312: 283–289, 1985. doi: 10.1056/NEJM198501313120505. [DOI] [PubMed] [Google Scholar]
- 14.Fakler B, Schultz JH, Yang J, Schulte U, Brandle U, Zenner HP, Jan LY, Ruppersberg JP. Identification of a titratable lysine residue that determines sensitivity of kidney potassium channels (ROMK) to intracellular pH. EMBO J 15: 4093–4099, 1996. doi: 10.1002/j.1460-2075.1996.tb00784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedman PA, Bushinsky DA. Diuretic effects on calcium metabolism. Semin Nephrol 19: 551–556, 1999. [PubMed] [Google Scholar]
- 16.Giebisch G. Mechanisms of renal tubular acidification. Klin Wochenschr 64: 853–861, 1986. doi: 10.1007/BF01725558. [DOI] [PubMed] [Google Scholar]
- 17.Giebisch G. Renal potassium transport: mechanisms and regulation. Am J Physiol Renal Physiol 274: F817–F833, 1998. [DOI] [PubMed] [Google Scholar]
- 18.Glanville M, Kingscote S, Thwaites DT, Simmons NL. Expression and role of sodium, potassium, chloride cotransport (NKCC1) in mouse inner medullary collecting duct (mIMCD-K2) epithelial cells. Pflugers Arch 443: 123–131, 2001. doi: 10.1007/s004240100629. [DOI] [PubMed] [Google Scholar]
- 19.Grimm PR, Foutz RM, Brenner R, Sansom SC. Identification and localization of BK-β subunits in the distal nephron of the mouse kidney. Am J Physiol Renal Physiol 293: F350–F359, 2007. doi: 10.1152/ajprenal.00018.2007. [DOI] [PubMed] [Google Scholar]
- 20.Grimm PR, Lazo-Fernandez Y, Delpire E, Wall SM, Dorsey SG, Weinman EJ, Coleman R, Wade JB, Welling PA. Integrated compensatory network is activated in the absence of NCC phosphorylation. J Clin Invest 125: 2136–2150, 2015. doi: 10.1172/JCI78558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grimm PR, Sansom SC. BK channels in the kidney. Curr Opin Nephrol Hypertens 16: 430–436, 2007. doi: 10.1097/MNH.0b013e32826fbc7d. [DOI] [PubMed] [Google Scholar]
- 22.Ho K, Nichols CG, Lederer WJ, Lytton J, Vassilev PM, Kanazirska MV, Hebert SC. Cloning and expression of an inwardly rectifying ATP-regulated potassium channel. Nature 362: 31–38, 1993. doi: 10.1038/362031a0. [DOI] [PubMed] [Google Scholar]
- 23.Holtzclaw JD, Grimm PR, Sansom SC. Intercalated cell BK-alpha/beta4 channels modulate sodium and potassium handling during potassium adaptation. J Am Soc Nephrol 21: 634–645, 2010. doi: 10.1681/ASN.2009080817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hropot M, Fowler N, Karlmark B, Giebisch G. Tubular action of diuretics: distal effects on electrolyte transport and acidification. Kidney Int 28: 477–489, 1985. doi: 10.1038/ki.1985.154. [DOI] [PubMed] [Google Scholar]
- 25.Huo R, Du T, Xu Y, Xu W, Chen X, Sun K, Yu X. Effects of Mediterranean-style diet on glycemic control, weight loss and cardiovascular risk factors among type 2 diabetes individuals: a meta-analysis. Eur J Clin Nutr 69: 1200–1208, 2015. doi: 10.1038/ejcn.2014.243. [DOI] [PubMed] [Google Scholar]
- 26.Jönsson T, Granfeldt Y, Ahrén B, Branell UC, Pålsson G, Hansson A, Söderström M, Lindeberg S. Beneficial effects of a Paleolithic diet on cardiovascular risk factors in type 2 diabetes: a randomized cross-over pilot study. Cardiovasc Diabetol 8: 35, 2009. doi: 10.1186/1475-2840-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kovacikova J, Winter C, Loffing-Cueni D, Loffing J, Finberg KE, Lifton RP, Hummler E, Rossier B, Wagner CA. The connecting tubule is the main site of the furosemide-induced urinary acidification by the vacuolar H+-ATPase. Kidney Int 70: 1706–1716, 2006. doi: 10.1038/sj.ki.5001851. [DOI] [PubMed] [Google Scholar]
- 28.Lau KK, Wong YK, Chan YH, Li OY, Sing Lee PY, Yuen GG, Wong YK, Tong S, Wong D, Chan KH, Cheung RT, Siu CW, Ho SL, Tse HF. Mediterranean-style diet is associated with reduced blood pressure variability and subsequent stroke risk in patients with coronary artery disease. Am J Hypertens, 28: 501–507, 2015. doi: 10.1093/ajh/hpu195. [DOI] [PubMed] [Google Scholar]
- 29.Lee CT, Chen HC, Lai LW, Yong KC, Lien YH. Effects of furosemide on renal calcium handling. Am J Physiol Renal Physiol 293: F1231–F1237, 2007. doi: 10.1152/ajprenal.00038.2007. [DOI] [PubMed] [Google Scholar]
- 30.Liu W, Schreck C, Coleman RA, Wade JB, Hernandez Y, Zavilowitz B, Warth R, Kleyman TR, Satlin LM. Role of NKCC in BK channel-mediated net K+ secretion in the CCD. Am J Physiol Renal Physiol 301: F1088–F1097, 2011. doi: 10.1152/ajprenal.00347.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malnic G, De Mello Aires M, Giebisch G. Potassium transport across renal distal tubules during acid-base disturbances. Am J Physiol Renal Physiol 221: 1192–1208, 1971. doi: 10.1152/ajplegacy.1971.221.4.1192. [DOI] [PubMed] [Google Scholar]
- 32.Martinez-Gonzalez MA, Bes-Rastrollo M. Dietary patterns, Mediterranean diet, and cardiovascular disease. Curr Opin Lipidol 25: 20–26, 2014. doi: 10.1097/MOL.0000000000000044. [DOI] [PubMed] [Google Scholar]
- 33.McNicholas CM, MacGregor GG, Islas LD, Yang Y, Hebert SC, Giebisch G. pH-dependent modulation of the cloned renal K+ channel, ROMK. Am J Physiol Renal Physiol 275: F972–F981, 1998. doi: 10.1152/ajprenal.1998.275.6.F972. [DOI] [PubMed] [Google Scholar]
- 34.Na KY, Kim GH, Joo KW, Lee JW, Jang HR, Oh YK, Jeon US, Chae SW, Knepper MA, Han JS. Chronic furosemide or hydrochlorothiazide administration increases H+-ATPase B1 subunit abundance in rat kidney. Am J Physiol Renal Physiol 292: F1701–F1709, 2007. doi: 10.1152/ajprenal.00270.2006. [DOI] [PubMed] [Google Scholar]
- 35.Pácha J, Frindt G, Sackin H, Palmer LG. Apical maxi K channels in intercalated cells of CCT. Am J Physiol Renal Physiol 261: F696–F705, 1991. doi: 10.1152/ajprenal.1991.261.4.F696. [DOI] [PubMed] [Google Scholar]
- 36.Palmer LG, Choe H, Frindt G. Is the secretory K channel in the rat CCT ROMK? Am J Physiol Renal Physiol 273: F404–F410, 1997. doi: 10.1152/ajprenal.1997.273.3.F404. [DOI] [PubMed] [Google Scholar]
- 37.Pech V, Pham TD, Hong S, Weinstein AM, Spencer KB, Duke BJ, Walp E, Kim YH, Sutliff RL, Bao HF, Eaton DC, Wall SM. Pendrin modulates ENaC function by changing luminal HCO3−. J Am Soc Nephrol 21: 1928–1941, 2010. doi: 10.1681/ASN.2009121257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quinn SJ, Bai M, Brown EM. pH Sensing by the calcium-sensing receptor. J Biol Chem 279: 37241–37249, 2004. doi: 10.1074/jbc.M404520200. [DOI] [PubMed] [Google Scholar]
- 39.Rastogi SP, Crawford C, Wheeler R, Flanigan W, Arruda JA. Effect of furosemide on urinary acidification in distal renal tubular acidosis. J Lab Clin Med 104: 271–282, 1984. [PubMed] [Google Scholar]
- 40.Reeuwijk HJ, Tjaden UR, van der Greef J. Simultaneous determination of furosemide and amiloride in plasma using high-performance liquid chromatography with fluorescence detection. J Chromatogr A 575: 269–274, 1992. doi: 10.1016/0378-4347(92)80155-J. [DOI] [PubMed] [Google Scholar]
- 41.Renkema KY, Velic A, Dijkman HB, Verkaart S, van der Kemp AW, Nowik M, Timmermans K, Doucet A, Wagner CA, Bindels RJ, Hoenderop JG. The calcium-sensing receptor promotes urinary acidification to prevent nephrolithiasis. J Am Soc Nephrol 20: 1705–1713, 2009. doi: 10.1681/ASN.2008111195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reyes AJ. Effects of diuretics on outputs and flows of urine and urinary solutes in healthy subjects. Drugs 41, Suppl 3: 35–59, 1991. doi: 10.2165/00003495-199100413-00006. [DOI] [PubMed] [Google Scholar]
- 43.Reyes AJ, Taylor SH. Diuretics in cardiovascular therapy: the new clinicopharmacological bases that matter. Cardiovasc Drugs Ther 13: 371–398, 1999. doi: 10.1023/A:1007835821228. [DOI] [PubMed] [Google Scholar]
- 44.Riccardi D, Valenti G. Localization and function of the renal calcium-sensing receptor. Nat Rev Nephrol 12: 414–425, 2016. doi: 10.1038/nrneph.2016.59. [DOI] [PubMed] [Google Scholar]
- 45.Rodicio JL, Hernando L. Effects and interactions of furosemide and acetazolamide on tubular function in rat kidney. Rev Esp Fisiol 33: 113–118, 1977. [PubMed] [Google Scholar]
- 46.Rubera I, Loffing J, Palmer LG, Frindt G, Fowler-Jaeger N, Sauter D, Carroll T, McMahon A, Hummler E, Rossier BC. Collecting duct-specific gene inactivation of alphaENaC in the mouse kidney does not impair sodium and potassium balance. J Clin Invest 112: 554–565, 2003. doi: 10.1172/JCI16956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sansom SC, Welling PA. Two channels for one job. Kidney Int 72: 529–530, 2007. doi: 10.1038/sj.ki.5002438. [DOI] [PubMed] [Google Scholar]
- 48.Shavit L, Chen L, Ahmed F, Ferraro PM, Moochhala S, Walsh SB, Unwin R. Selective screening for distal renal tubular acidosis in recurrent kidney stone formers: initial experience and comparison of the simultaneous furosemide and fludrocortisone test with the short ammonium chloride test. Nephrol Dial Transplant 31: 1870–1876, 2016. doi: 10.1093/ndt/gfv423. [DOI] [PubMed] [Google Scholar]
- 49.Shirley DG, Skinner J, Walter SJ. The influence of dietary potassium on the renal tubular effect of hydrochlorothiazide in the rat. Br J Pharmacol 91: 693–699, 1987. doi: 10.1111/j.1476-5381.1987.tb11264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stokes JB. Consequences of potassium recycling in the renal medulla. Effects of ion transport by the medullary thick ascending limb of Henle’s loop. J Clin Invest 70: 219–229, 1982. doi: 10.1172/JCI110609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Terker AS, Zhang C, Erspamer KJ, Gamba G, Yang CL, Ellison DH. Unique chloride-sensing properties of WNK4 permit the distal nephron to modulate potassium homeostasis. Kidney Int 89: 127–134, 2016. doi: 10.1038/ki.2015.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Topala CN, Schoeber JP, Searchfield LE, Riccardi D, Hoenderop JG, Bindels RJ. Activation of the Ca2+-sensing receptor stimulates the activity of the epithelial Ca2+ channel TRPV5. Cell Calcium 45: 331–339, 2009. doi: 10.1016/j.ceca.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 53.US Department of Health and Human Services; Centers for Disease Control; National Center for Health Statistics . Health, United States, 2015: With Special Feature on Racial and Ethnic Health Disparities. Hyattsville, MD: National Center for Health Statistics, 2016. [Google Scholar]
- 54.Walsh SB, Shirley DG, Wrong OM, Unwin RJ. Urinary acidification assessed by simultaneous furosemide and fludrocortisone treatment: an alternative to ammonium chloride. Kidney Int 71: 1310–1316, 2007. doi: 10.1038/sj.ki.5002220. [DOI] [PubMed] [Google Scholar]
- 55.Wang B, Wen D, Li H, Wang-France J, Sansom SC. Net K+ secretion in the thick ascending limb of mice on a low-Na, high-K diet. Kidney Int 92: 864–875, 2017. doi: 10.1016/j.kint.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang DD, Li Y, Chiuve SE, Hu FB, Willett WC. Improvements in US diet helped reduce disease burden and lower premature deaths, 1999-2012; overall diet remains poor. Health Aff (Millwood) 34: 1916–1922, 2015. doi: 10.1377/hlthaff.2015.0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weiner ID, Hamm LL. Regulation of Cl−/HCO3− exchange in the rabbit cortical collecting tubule. J Clin Invest 87: 1553–1558, 1991. doi: 10.1172/JCI115168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Welling PA. Roles and regulation of renal K channels. Annu Rev Physiol 78: 415–435, 2016. doi: 10.1146/annurev-physiol-021115-105423. [DOI] [PubMed] [Google Scholar]
- 59.Wen D, Cornelius RJ, Rivero-Hernandez D, Yuan Y, Li H, Weinstein AM, Sansom SC. Relation between BK-α/β4-mediated potassium secretion and ENaC-mediated sodium reabsorption. Kidney Int 86: 139–145, 2014. doi: 10.1038/ki.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wen D, Cornelius RJ, Sansom SC. Interacting influence of diuretics and diet on BK channel-regulated K homeostasis. Curr Opin Pharmacol 15: 28–32, 2014. doi: 10.1016/j.coph.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wen D, Cornelius RJ, Yuan Y, Sansom SC. Regulation of BK-α expression in the distal nephron by aldosterone and urine pH. Am J Physiol Renal Physiol 305: F463–F476, 2013. doi: 10.1152/ajprenal.00171.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wen D, Yuan Y, Warner PC, Wang B, Cornelius RJ, Wang-France J, Li H, Boettger T, Sansom SC. Increased epithelial sodium channel activity contributes to hypertension caused by Na+- HCO3− cotransporter electrogenic 2 deficiency. Hypertension 66: 68–74, 2015. doi: 10.1161/HYPERTENSIONAHA.115.05394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Widmer RJ, Flammer AJ, Lerman LO, Lerman A. The Mediterranean diet, its components, and cardiovascular disease. Am J Med 128: 229–238, 2015. doi: 10.1016/j.amjmed.2014.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Woda CB, Bragin A, Kleyman TR, Satlin LM. Flow-dependent K+ secretion in the cortical collecting duct is mediated by a maxi-K channel. Am J Physiol Renal Physiol 280: F786–F793, 2001. doi: 10.1152/ajprenal.2001.280.5.F786. [DOI] [PubMed] [Google Scholar]
- 65.Xie R, Xu J, Xiao Y, Wu J, Wan H, Tang B, Liu J, Fan Y, Wang S, Wu Y, Dong TX, Zhu MX, Carethers JM, Dong H, Yang S. Calcium Promotes human gastric cancer via a novel coupling of calcium-sensing receptor and TRPV4 channel. Cancer Res 77: 6499–6512, 2017. doi: 10.1158/0008-5472.CAN-17-0360. [DOI] [PubMed] [Google Scholar]