Abstract
Increased expression of vascular cell adhesion molecule 1 (VCAM-1) on the aortic endothelium is an early marker of atherogenesis, promoted in part by elevated levels of inflammatory cytokines such as TNF-α. Mammalian target of rapamycin (mTOR) is a ubiquitous signaling molecule that has been considered to contribute to diverse cellular processes through mTOR complex 1 (mTORC1) or complex 2 (mTORC2). This study aimed to elucidate the role of mTOR signaling in TNF-α-induced VCAM-1 expression by the arterial endothelium. Primary human aortic endothelial cells (HAECs) were treated with low-dose (0.1 ng/ml) TNF-α, and VCAM-1 expression was measured by real-time quantitative PCR, Western blot analysis, and flow cytometry. Inhibition of mTOR through siRNA-mediated depletion or treatment with chemical inhibitors rapamycin or torin 1 suppressed VCAM1 transcription, which translated to inhibition of VCAM-1 surface expression by HAECs and concomitant decreased adhesion of monocytes. A promoter luciferase assay and chromatin immunoprecipitation indicated that mTOR regulated VCAM1 transcription through a mechanism involving transcription factor GATA6. Activation of PKC-α and an increase in miR-200a-3p expression, caused by mTOR inhibition but not disruption of mTORC1 or mTORC2 singly or together, decreased TNF-α-induced GATA6 expression and its enrichment at the VCAM1 promoter. In conclusion, mTOR inhibition activates PKC-α independently of disruption of mTORC1 and/or mTORC2, which challenges the conventional wisdom regarding mTOR signaling. Moreover, mTOR signals through transcriptional and posttranscriptional mechanisms to elicit maximal cytokine-induced endothelial inflammation that precedes atherosclerosis.
NEW & NOTEWORTHY Both mammalian target of rapamycin (mTOR) complex 1 (mTORC1) and mTORC2 contribute to PKC-α activation in the human aortic endothelium. Inhibition of mTOR is not equivalent to disruption of mTORC1 and/or mTORC2 in affecting human aortic endothelial cell signaling. Specifically, inhibition of mTOR causes PKC-α activation and miR-200a-3p upregulation, which independently suppresses TNF-α-induced transcription factor GATA6 expression and subsequently inhibits VCAM-1 expression and monocytic cell adhesion onto the aortic endothelium.
Keywords: atherosclerosis, endothelium, GATA6, vascular cell adhesion molecule 1
INTRODUCTION
Atherosclerosis, a chronic inflammatory response of the arterial wall, is the main pathology underlying cardiovascular diseases, which are the leading causes of mortality and morbidity worldwide. Endothelial dysfunction marked by upregulated expression of cellular adhesion molecules on the endothelial surface in lesion-prone areas (13) is central to atherogenesis (4). Specifically, vascular cell adhesion molecule 1 (VCAM-1) plays a central role in the initiation of atherosclerosis (7) by mediating firm adhesion of activated monocytes to the arterial wall via binding with α4β1-integrin (21).
Mammalian target of rapamycin (mTOR) is a constitutively active serine/threonine kinase that interacts with several proteins to form two distinct complexes: mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2) (19). In addition to mTOR, these two complexes share target of rapamycin complex subunit LST8 (mLST8), DEP domain-containing mTOR-interacting protein (Deptor), and the TELO2-interacting protein-1 (Tti1)/telomere length regulation protein TEL2 (Tel2) complex. mTORC1, specifically containing regulatory-associated protein of mTOR (Raptor) and proline-rich Akt/PKB substrate 40 kDa (Pras40), regulates protein synthesis and cell proliferation and growth through phosphorylation of downstream effectors S6 kinase-1 and 4E-binding protein-1 (4EBP1) (19). Rapamycin-insensitive companion of mTOR (Rictor), mammalian stress-activated protein kinase-interaction protein 1 (mSin1), and protein observed with Rictor-1/2 (Protor1/2) are specific to mTORC2, which is considered to be responsible for the regulation of cytoskeletal organization and cell survival through activation of members of the AGC kinase subfamily such as protein kinase C-α (PKC-α) (30), protein kinase B (Akt), and serum-and glucocorticoid-induced kinase-1 (SGK1) (19). Moreover, mTORC2 has been implicated in protein maturation and stability (14).
In addition to controlling mRNA translation and protein stability, mTOR signaling was recently found to be associated with regulation of noncoding RNA, including microRNAs (miRNAs). By regulating gene expression through binding to the 3′-untranslated region of target mRNA, miRNAs are involved in diverse processes such as lipid metabolism and affect the development of atherosclerosis (25). Moreover, they play fundamental roles in maintaining endothelial homeostasis and controlling leukocyte trafficking and inflammation (10). Disruption of either mTORC1 or both mTORC1 and mTORC2 caused remarkable changes in expression of diverse miRNAs leading to modification of cellular processes (29, 41).
Given the involvement of mTOR signaling in a diversity of physiological and pathological cellular processes such as organismal growth, cancer, type 2 diabetes, and neurodegeneration (19), chemical inhibitors have been widely applied as disease interventions. Rapamycin, also known as sirolimus, is a natural anti-inflammatory macrolide used as an immunosuppressant in autoimmune diseases or after organ transplantation and for the treatment of cancer and prevention of coronary artery in-stent restenosis (19). So far, mTOR is the only known target for rapamycin that binds to FK-506 inside the cell and acutely inhibits mTORC1 by disrupting the association between mTOR and Raptor (26). It has been shown that chronic treatment with rapamycin also disrupts mTOR2 signaling (31). In addition to rapamycin, a new class of ATP-competitive mTOR inhibitors, such as torin 1, which inhibits both mTOR complexes, have been synthesized and approved for clinical trials for cancer (22, 39).
In animal models of atherosclerosis such as apolipoprotein E-deficient (5) and LDL receptor-deficient (24) mice or hypercholesterolemic rabbits (1), administration of high-dose rapamycin or its analog everolimus results in decreased plaque size, suggesting a beneficial effect of inhibition of mTOR on the vasculature and the necessity to explore the underlying cell-specific mechanisms. mTORC1 in macrophages has been found to contribute to chemokine (C-C motif) ligand 2 expression and atherosclerosis. However, the effect of mTOR signaling on cytokine-induced adhesion molecule expression by endothelial cells has rarely been studied (36). Notable is the paucity of studies to investigate the regulation of mTOR signaling in the arterial endothelium and how it contributes to the early inflammatory responses that promote atherosclerosis.
In the present study, we evaluated a role for mTOR in signaling TNF-α-induced VCAM-1 expression in primary human aortic endothelial cells (HAECs). We report that inhibition of mTOR significantly suppressed TNF-α-induced VCAM1 transcription through a mechanism involving the transcription factor GATA6. Contrary to the accepted view that mTOR regulates PKC-α activation via mTORC2 (30), both mTORC1 and mTORC2 were required for PKC-α activation in HAECs. Inhibition of mTOR increased, rather than attenuated, phosphorylation of PKC-α. The increase in PKC-α activity and miR-200a-3p expression caused by inhibition of mTOR independently decreased GATA6-mediated VCAM-1 expression and monocytic cell adhesion to HAEC monolayers.
MATERIALS AND METHODS
Cell culture and treatment.
Primary HAECs were purchased from the American Type Culture Collection (catalog no. PCS-100-011, lot no. 63233442, Manassas, VA) and maintained in Endothelial Growth Medium-2 (Lonza) supplemented with 10% FBS (GIBCO). Unless otherwise indicated, HAECs up to passage 8 without serum starvation were pretreated with the mTOR inhibitors rapamycin (25 nM, Enzo Life Sciences) or torin 1 (75 nM, Cell Signaling Technology) for 16 h or with the PKC inhibitor GF109203X (10 nM or 10 μM, Sigma) or activator PMA (1 μM, Sigma) for 1 h. Cells were then stimulated with 0.1 ng/ml TNF-α (R&D Systems) for 2 h for mRNA measurement or for 4 h before Western blot or flow cytometry analyses.
Cell transfection.
For siRNA (all from Santa Cruz Biotechnology), a 4D-Nucleofector system (Lonza) was used to transfect HAECs (32). For miRNA mimic, interferon regulatory factor 1 (IRF-1) and GATA luciferase reporter plasmids, and the VCAM1 promoter luciferase constructs, transfection was performed using Lipofectamine 2000 (ThermoFisher Scientific). At 48–96 h posttransfection, HAECs were further treated and analyzed.
Western blot analysis.
The Western blot procedure was performed as previously described (32). Briefly, HAECs were lysed, and protein concentrations were measured. Proteins were separated with SDS-PAGE and transferred to a PVDF membrane. After membranes had been blocked and incubated with primary and horseradish peroxidase-conjugated secondary antibodies, the target protein band was visualized with ECL (Pierce) and a digital gel image-analysis system. Primary antibodies against VCAM-1, NF-κB, phospho-Ser63 c-Jun, phospho-Ser657 PKC-α, and PKC-α were from Santa Cruz Biotechnology. Antibodies for GAPDH, mTOR, Rictor, Raptor, phospho-Ser536 NF-κB, c-Jun IRF-1, GATA6, phospho-Ser389 70-kDa ribosomal protein S6 kinase (p70S6K), p70S6K, phospho-Ser473 Akt, Akt, and α-tubulin were from Cell Signaling Technology. Band densities were analyzed with ImageJ software (National Institutes of Health). Shown in this study are representative images from at least three independent experiments.
Flow cytometry.
HAECs were detached using an enzyme-free cell dissociation buffer (GIBCO) and labeled with FITC-conjugated anti-human VCAM-1 antibody or PerCP-Cy5.5-conjugated anti-human ICAM-1 antibody (BD Pharmingen, BD Biosciences) (32). Cells were then submitted for analysis by a FACSCalibur flow cytometer (BD Biosciences). FlowJo software was applied for postacquisition analysis.
Monocyte adhesion.
Fluorescent dye DiO-labeled THP-1 cells (5 × 104, American Type Culture Collection) were cocultured with TNF-α-stimulated HAECs in a 12-well plate for 10 min. After three washes with PBS, THP-1 cells adhered onto a HAEC monolayer were identified by positive DiO fluorescence under a fluorescence microscope.
Immunofluorescent staining.
HAECs were grown on coverslips to confluence before fixation, permeabilization, and staining with rabbit anti-human GATA6 antibody (Cell Signaling Technology). After incubation with FITC-conjugated anti-rabbit IgG, cells were counterstained with DAPI. Images were captured with a fluorescence microscope (Olympus IX53), and FITC intensity of cells was quantified with Image-Pro Plus 6.0 (Media Cybernetics).
Quantification of mRNA and miRNA.
After treatment, total RNA in HAECs was extracted using TRIzol reagent. To quantify mRNA, total RNA was reverse transcribed into cDNA using the PrimeScript RT kit (Takara Biotechnology) followed by real-time PCR using SYBR Green (Roche) on a LightCycler 480 Instrument II (Roche). Relative gene expression was normalized to the GAPDH mRNA level with the method (where Ct is threshold cycle). The primers for mRNA measurement were as follows: GATA6, forward 5′-TCAAACCAGGAAACGAAAACC-3′ and reverse 5′-TTGGAGTCATGGGAATGGAAT-3′; VCAM-1, forward 5′-AACCTTGCAGCTTACAGTGA-3′ and reverse 5′-TGTGTGAAGGAGTTAATTTGATTGG-3′; and GAPDH, forward 5′-GGATTTGGTCGTATTGGG-3′ and reverse 5′-GGAAGATGGTGATGGGATT-3′, respectively. The primers used to measure heterogeneous nuclear RNA of VCAM1 were as follows: forward 5′-CACTGCTTTGATTCCCTTCTCTTTGGAG-3′ and reverse 5′-ATGCAAAATAGAGCACGAGAAGCTCAGG-3′. To quantify miR-181b-5p, miR-181d-5p, miR-200a-3p, and miR-196-5p, the PrimeScript RT kit and specific miRNA primer sets (RiboBio) were used for reverse transcription and real-time PCR. Relative expression was normalized to U6 levels.
Nuclear protein extraction.
After the appropriate treatment, nuclear protein was extracted from HAECs using a Nuclear and Cytoplasmic Protein Extraction kit (Beyotime). The quality of the nuclear extraction was confirmed with Western blot detection of lamin B1.
Luciferase activity assay.
VCAM1 −1,641/+12, −288/+12, and −228/+12 were amplified by PCR using forward and reverse primers containing restriction sites for MluI and XhoI and inserted into pGL3 firefly luciferase reporter vector (Promega) (8). The Site-Directed Mutagenesis Kit (Agilent) was used to generate VCAM1 −288/+12 GATA mut (with the GATA −259 site mutated) and VCAM1 −288/+12 IRF-1 mut (with the IRF-1 binding site mutated). HAECs growing on a 24-well plate were transfected with 0.8 μg of each construct using Lipofectamine 2000 together with 0.1 μg phRL-TK (Promega) containing Renilla luciferase cistron, which served as an internal transfection control. Luciferase activity was measured with Dual-Luciferase Reporter system (Promega) on a GloMax 20/20 Luminometer (Promega).
Chromatin immunoprecipitation assay.
HAECs were pretreated with or without rapamycin (25 nM) for 16 h followed by a second 2-h incubation with TNF-α (1 ng/ml). The preparation and immunoprecipitation of HAEC chromatin were performed using the ChIP-IT Express kit (Active Motif). VCAM1 promoter binding to GATA6 was quantified by real-time PCR and normalized to input DNA.
Statistical analysis.
GraphPad Prism was used for data presentation and statistical analysis. Data are presented as means ± SE. A Student’s t-test was used to compare the difference between two groups, whereas ANOVA with a Dunnett’s test or Newman-Keuls posttest was applied to analyze multiple groups. P ≤ 0.05 was considered significant.
RESULTS
Inhibition of mTOR reduced TNF-α-induced VCAM-1 expression and monocyte adhesion onto HAECs.
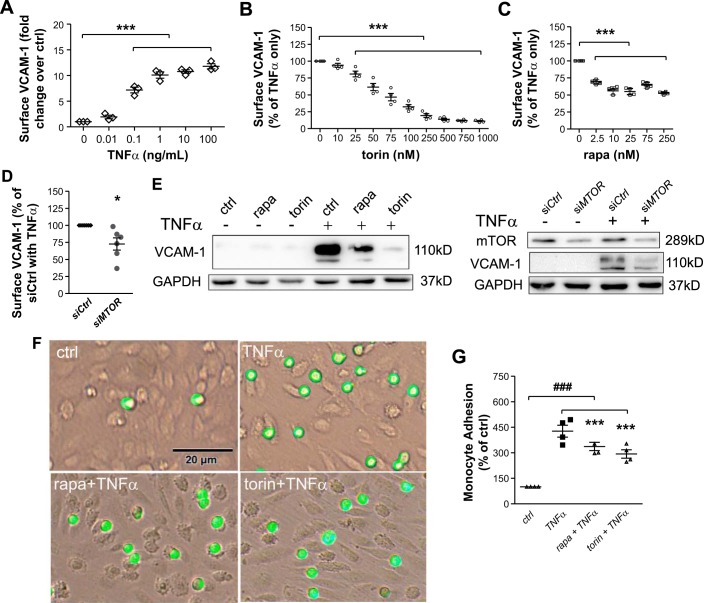
Cytokines such as TNF-α are elevated in the proinflammatory milieu of atherosclerosis and contribute to its progression by potent induction of VCAM-1 (3). To demonstrate the responsiveness of HAECs to TNF-α while establishing an effective dose for subsequent experiments, we measured surface expression of the adhesion molecules VCAM-1 and ICAM-1 by flow cytometry. Expression increased in a dose-dependent manner at a range from 0.01 to 100 ng/ml, plateauing above 1 ng/ml, with an EC50 value of ~0.1 ng/ml (Fig. 1A). At this concentration, TNF-α elevated transcription of VCAM1 and ICAM1 to 9.5- and 8.7-fold, respectively, at 2 h and HAEC surface expression to 7.0- and 8.0-fold, respectively, at 4 h (data not shown). The observed EC50 value was similar to that previously observed (32) and was applied to treatments throughout this study.
Fig. 1.
Pharmacological inhibition or depletion of mammalian target of rapamycin (mTOR) attenuates TNF-α-induced VCAM-1 expression and monocyte adhesion onto human aortic endothelial cells (HAECs). A: HAECs were treated with TNF-α at the indicated dose for 4 h followed by flow cytometric analysis of VCAM-1 surface expression (n = 3). B and C: after 16-h pretreatment with torin 1 (B) or rapamycin (rapa; C) at the indicated concentrations, HAECs were stimulated with 0.1 ng/ml TNF-α for 4 h followed by flow cytometric analysis of VCAM-1 surface expression (n = 4). D: HAECs were transfected with MTOR-targeted siRNA (siMTOR) and stimulated with TNF-α for 4 h followed by flow cytometric analysis of VCAM-1 expression (n = 6). E: HAECs were pretreated with 75 nM torin 1 or 25 nM rapamycin or transfected with MTOR-targeted siRNA and stimulated with 0.1 ng/ml TNF-α for 4 h followed by Western blot analysis of VCAM-1 expression (n = 6). Shown are representative blots. F and G: HAECs were pretreated with rapamycin or torin 1 followed by stimulation with TNF-α as described above. Adhered DiO-stained THP-1 cells were visualized by fluorescence microscopy (F) and quantified (G) (n = 4). Values are means ± SE. ctrl, control; siCtrl, control siRNA. *P ≤ 0.05 and ***P ≤ 0.0001 vs. TNF-α; ###P ≤ 0.0001 vs. ctrl (one-way repeated-measures ANOVA followed by Dunnett’s test, A–C and G; two-tailed paired t-test, D).
Treatment with torin 1, an inhibitor of both mTORC1 and mTORC2 (22), decreased TNF-α-induced VCAM-1 surface expression on HAECs in a dose-dependent manner (Fig. 1B). The minimal effective dose was 25 nM. Torin 1 elicited an ~50% reduction in VCAM-1 expression at 75 nM. Similarly, treatment with rapamycin for 16 h inhibited both mTORC1 and mTORC2 as evidenced by diminished phosphorylation of p70S6K and decreased phosphorylation of Akt at Ser473 (data not shown). A reduction in VCAM-1 expression resulted at the minimal effective dose of 2.5 nM, and peak inhibition was observed at ~25 nM rapamycin (Fig. 1C). Consistent with pharmacological inhibition, knockdown of mTOR via siRNA attenuated TNF-α-induced cell surface expression of VCAM-1 by 27.2% (Fig. 1D). Inhibition of mTOR either by rapamycin or torin 1 or by MTOR siRNA caused a similar reduction in the total amount of VCAM-1 protein as revealed by Western blot analysis (Fig. 1E). Consistent with the critical function of VCAM-1 in mediating monocyte adhesion, inhibition of mTOR significantly suppressed adhesion of monocytic THP-1 cells onto TNF-α-inflamed HAEC monolayers (Fig. 1, F and G).
Inhibition of mTOR, but not disruption of mTORC1 or mTORC2 singly or in tandem, significantly suppressed VCAM1 transcription.
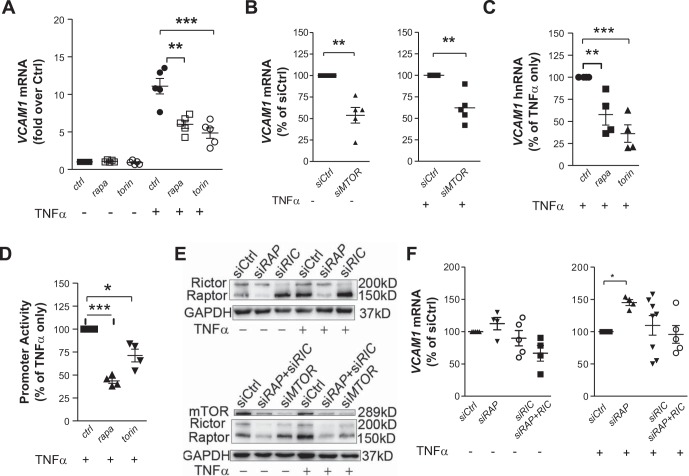
Real-time quantitative PCR indicated that 25 nM rapamycin or 75 nM torin 1 decreased TNF-α-induced VCAM1 steady-state mRNA by 37.8% and 51.4%, respectively (Fig. 2A), and siRNA-mediated knockdown of mTOR attenuated it by 38.5% (Fig. 2B). A similar reduction in VCAM1 heterogeneous nuclear RNA (Fig. 2C) confirmed that inhibition of mTOR affected VCAM1 transcription itself rather than the stability of the transcription product. This inhibition was attributed to decreased VCAM1 promoter activity as demonstrated with the Dual-Luciferase Reporter assay. Rapamycin or torin 1 treatment significantly decreased the promoter activity of VCAM1 −1,641/+12 (relative to the start site of transcription) construct (Fig. 2D).
Fig. 2.
Inhibition of mammalian target of rapamycin (mTOR) but not disruption of mTOR complex 1 and/or complex 2 suppresses TNF-α-induced VCAM1 transcription in human aortic endothelial cells (HAECs). A–C: HAECs were pretreated with 75 nM torin 1 or 25 nM rapamycin (rapa) or transfected with MTOR-targeted siRNA (siMTOR) and stimulated with 0.1 ng/ml TNF-α for 2 h followed by quantitative PCR to measure steady-state VCAM1 mRNA (n = 5; A and B) or heterogeneous nuclear RNA (hnRNA, n = 4; C). D: activity of the VCAM1 promoter (−1,641/+12) construct was measured by the Dual-Luciferase Reporter system (n = 4). E: Western blot analysis confirmed successful knockdown by siRNA (n = 8). Shown are representative blots. F: 48–72 h after transfection, HAECs were treated with or without 0.1 ng/ml TNF-α for 2 h before real-time PCR to quantify VCAM1 mRNA (n = 4–7). Values are means ± SE. ctrl, control; siCtrl, control siRNA; siRAP, regulatory-associated protein of mTOR (RAPTOR)-targeted siRNA; siRIC, rapamycin-insensitive companion of mTOR (RICTOR)-targeted siRNA. *P ≤ 0.05; **P ≤ 0.001; ***P ≤ 0.0001 (one-way ANOVA followed by Dunnett’s test, A, C, D, and F; two-tailed paired t-test, B).
To evaluate the role of mTORC1 and mTORC2 in the regulation of VCAM1 transcription in HAECs, the unique components of these two complexes, Raptor and Rictor, were depleted separately or together. Depletion of neither Raptor nor Rictor significantly decreased the VCAM1 mRNA level (Fig. 2, E and F). On the contrary, knockdown of Raptor increased it in the presence of TNF-α. Surprisingly, combined depletion of Rictor and Raptor also did not affect TNF-α-induced VCAM1 transcription (Fig. 2, E and F). These results suggest that inhibition of mTOR suppressed VCAM1 transcription through mechanisms that could not be attributed to disruption of mTORC1 or mTORC2 singly or in tandem.
Decreased GATA6 activity underlies VCAM1 transcriptional suppression caused by mTOR inhibition.
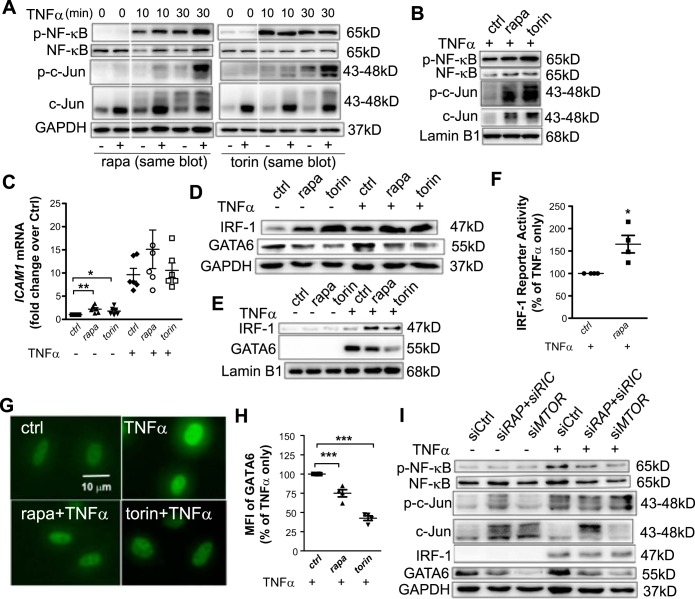
To investigate transcriptional regulation of VCAM1 by mTOR, the activity of VCAM1-binding transcription factors was examined in HAECs treated with rapamycin or torin 1 (Fig. 3, A, B, and D–H) or transfected with mTOR siRNA (Fig. 3I). As previously reported (18), TNF-α elicited a dynamic activation of transcription factors such as NF-κB p65 and c-Jun, an activator protein-1 component, with peaks at 10 and 30 min, respectively. Pretreatment with rapamycin or torin 1 did not decrease TNF-α-induced phosphorylation of NF-κB p65 or c-Jun at 10 or 30 min (Fig. 3, A and B) or at 4 h (data not shown). Rather, c-Jun phosphorylation was elevated in both the whole cell lysate (Fig. 3A) and nuclear extract (Fig. 3B) concomitant with activation of upstream JNK1/2 (data not shown). Similar results were observed in HAECs transfected with mTOR siRNA (Fig. 3I). These findings do not explain the transcriptional suppression of VCAM1 by inhibition of mTOR.
Fig. 3.
Decreased GATA6 expression is associated with inhibition of mammalian target of rapamycin (mTOR). A: 16 h after pretreatment with 75 nM torin 1 or 25 nM rapamycin (rapa), human aortic endothelial cells (HAECs) were stimulated with TNF-α for the indicated time followed by Western blot analysis for transcription factors (n = 3). The 0-, 10-, and 30-min TNF-α treatments were in the same blot. B: HAECs were pretreated with torin 1 or rapamycin and stimulated with TNF-α for 30 min. The nuclear extract was submitted for Western blot analysis of transcription factors (n = 3). C: HAECs were treated as described in Fig. 2A and ICAM1 mRNA was quantified (n = 5–6). D and E: HAECs were pretreated with torin 1 or rapamycin and stimulated with TNF-α for 4 h followed by Western blot analysis of the whole cell lysate (D) or nuclear extract (E) for interferon regulatory factor 1 (IRF-1) and GATA6 (n = 3). F: IRF-1 luciferase reporter activity was measured by the Dual-Luciferase Reporter system (n = 4). G and H: HAECs were grown on coverslips and immunolabeled for GATA6. Images were obtained by fluorescence microscopy (G) and nuclear intensity quantified (H) (n = 4). I: HAECs were transfected with siRNA and stimulated with TNF-α for 4 h followed by Western blot analysis of transcription factors (n = 5). Values are means ± SE. ctrl, control; MFI, mean fluorescence intensity; p-, phosphorylated; siCtrl, control siRNA; siMTOR, mTOR-targeted siRNA; siRAP, regulatory-associated protein of mTOR (RAPTOR)-targeted siRNA; siRIC, rapamycin-insensitive companion of mTOR (RICTOR)-targeted siRNA. *P ≤ 0.05; **P ≤ 0.001; ***P ≤ 0.0001 (one-way ANOVA followed by Dunnett’s test, C and H; two-tailed paired t-test, F).
In contrast to VCAM1, ICAM1 transcription was increased with torin 1 or rapamycin treatment, especially in the absence of TNF-α (Fig. 3C). Compared with the ICAM1 gene, the VCAM1 promoter contains unique binding sites for the transcription factors IRF-1 and GATA, which account for differential regulation of VCAM-1 and ICAM-1 expression (8, 32, 34, 37). Among the several GATAs in endothelial cells, GATA6 is TNF-α inducible and contributes significantly to its induction of VCAM-1 (35).
Western blot analysis indicated that treatment with rapamycin or torin 1 significantly increased IRF-1 expression and its localization in the nuclei (Fig. 3, D and E), leading to an increase in its transcriptional activity in HAECs, as indicated by a luciferase reporter assay (Fig. 3F). Although not as prominent as in the case of rapamycin or torin 1 treatment, a slight upregulation of IRF-1 was also observed in mTOR knockdown in the presence of TNF-α (Fig. 3I). These results excluded the possibility that rapamycin inhibited VCAM1 transcription through downregulation of IRF-1, as previously reported in venous endothelial cells (36). However, torin 1 or rapamycin treatment decreased GATA6 expression (Fig. 3D) and its localization in the nuclei (Fig. 3E) in cells stimulated with TNF-α. DAPI staining confirmed that GATA6 was exclusively localized in the nucleus (data not shown). Consistently, knockdown of MTOR but not combined depletion of Raptor and Rictor significantly decreased GATA6 expression in either the presence or absence of TNF-α (Fig. 3I). Rapamycin or torin 1 treatment significantly attenuated TNF-α-induced nuclear GATA6 expression (Fig. 3, G and H). These data implicate decreased GATA6 activity in transcriptional inhibition of VCAM1 caused by mTOR inhibition.
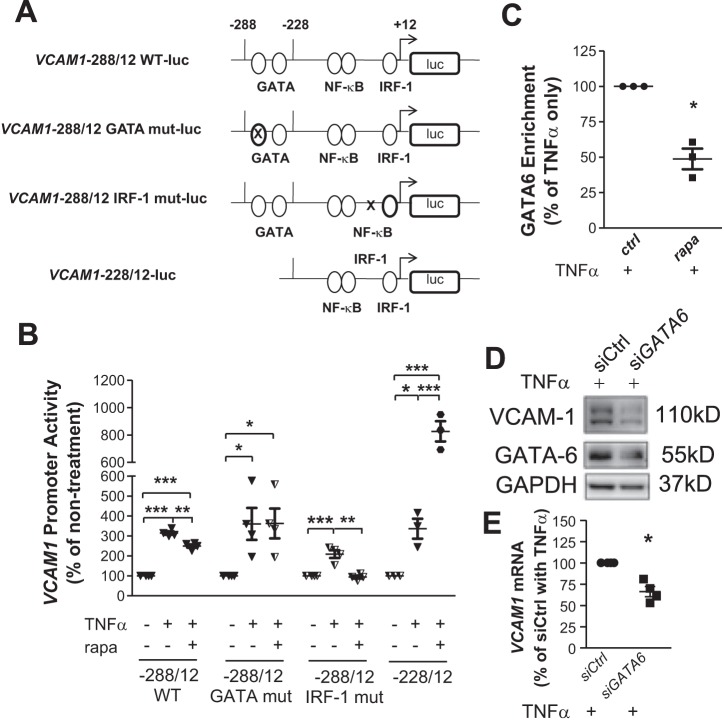
GATA and IRF-1 binding sites on the human VCAM1 gene promoter region are located between −288 and +12 bp relative to the start site of transcription (8). Among the two consensus GATA-binding sites at −244 and −259 bp, the latter is the main sequence used by endothelial cells for binding GATA in response to TNF-α (35). Accordingly, the following luciferase reporter constructs of three mutations along with the wild-type (WT) sequence (Fig. 4A) targeting cis elements of this region were created: −288/+12 WT, −228/+12 (without GATA-binding sites), −288/+12 GATA mut (with the GATA −259 site mutated), and −288/+12 IRF-1 mut (with the IRF-1 site mutated). The three mutations led to decreased basal promoter activity compared with WT (data not shown). Stimulation with TNF-α led to a 3.4-fold increase in the promoter activity of the −288/+12 WT construct (Fig. 4B), which was decreased by 33.3% in the presence of rapamycin. Mutation of the IRF-1 site effectively attenuated TNF-α-induced promoter activity. In addition, this mutation caused a further reduction when cells were treated with rapamycin (Fig. 4B), indicating involvement of an additional transcription factor/regulatory element underlying the inhibitory action of rapamycin. This was corroborated by the observation that activity of neither −228/+12 nor −288/+12 GATA mut constructs was decreased by rapamycin (Fig. 4C). In contrast, rapamycin treatment increased the activity of −228/+12, consistent with its positive effect on IRF-1 expression (Fig. 3, D and E, and Fig. 4B).
Fig. 4.
Reduced GATA6 activity underlies transcriptional inhibition of VCAM1 expression caused by mammalian target of rapamycin inhibition. A: firefly luciferase (luc) constructs of the VCAM1 promoter (−288/+12) were generated based on the cis elements for known binding transcription factors. Mutation (mut) is depicted by an “X.” B: human aortic endothelial cells (HAECs) were treated and activity of the constructs measured as previously described (n = 3–4). C: HAECs were incubated with or without 25 nM rapamycin (rapa) for 16 h followed by 2-h treatment with 1 ng/ml TNF-α. A chromatin immunoprecipitation assay was applied to examine enrichment of GATA6 onto the VCAM1 promoter (n = 3). D and E: HAECs were transfected with GATA6-targeted siRNA (siGATA6) followed by treatment with TNF-α for 4 h for Western blot analysis of VCAM-1 (D) or 2 h for quantitative PCR (E) (n = 4). Values are means ± SE. ctrl, control; IRF-1, interferon regulatory factor 1; siCtrl, control siRNA; WT, wild type. *P ≤ 0.05; **P ≤ 0.001; ***P ≤ 0.0001 (one-way ANOVA followed by a Newman-Keuls test, B; two-tailed paired t-test, C and E).
A chromatin immunoprecipitation assay was performed to confirm the involvement of GATA6. Consistent with our previous study in which TNF-α at 0.1 ng/ml provided insufficient stimulation to enrich the binding of IRF-1 to the VCAM1 promoter to a detectable level in a chromatin immunoprecipitation assay (32), the enrichment of GATA6 to VCAM1 was not detectable at this low concentration of TNF-α (data not shown). Therefore, 1 ng/ml TNF-α was applied to amplify promoter activity as in the previous study (32). Flow cytometry demonstrated that 25 nM rapamycin reduced 1 ng/ml TNF-α-induced VCAM-1 expression by 44.0%, which was comparable to the 37.0% decrease observed in parallel experiments with 0.1 ng/ml TNF-α treatment (data not shown) or 45.0% decrease in the previous experiment (Fig. 1B). Chromatin immunoprecipitation revealed that TNF-α stimulation resulted in a 2.2-fold increase in GATA6 binding to the VCAM1 promoter (data not shown). This binding was decreased by 49.8% with the treatment of rapamycin (Fig. 4C). Consistent with the known role of GATA6 in VCAM-1 expression (20, 34), knockdown of GATA6 with siRNA significantly attenuated TNF-α-induced VCAM1 transcription and consequent translation of protein (Fig. 4D). Together, these results clearly demonstrate that mTOR inhibition caused decreased GATA6 activity, which led to VCAM1 transcriptional repression.
Inhibition of mTOR was not equivalent to disruption of mTORC1 and/or mTORC2 in affecting HAEC signaling.
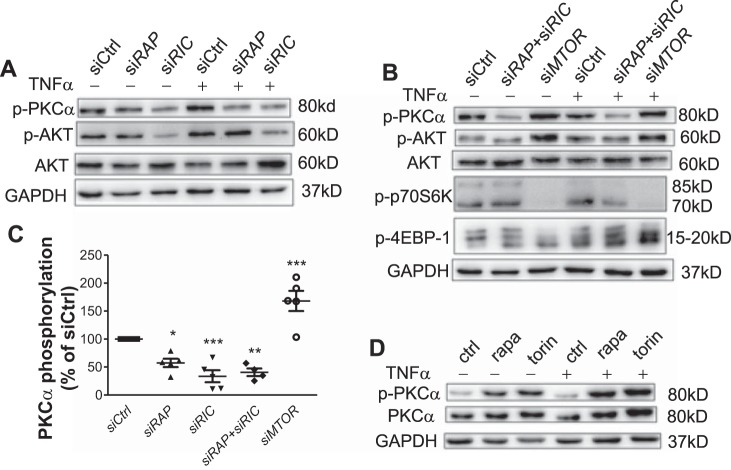
Downstream signaling was examined to further elucidate the mechanisms underlying the regulation of mTOR signaling on GATA6-mediated VCAM-1 expression. Consistent with the reported role of mTORC1 and mTORC2 (19), depletion of Raptor to disrupt mTORC1 inhibited phosphorylation of p70S6K and 4EBP1 (data not shown), whereas disruption of mTORC2 by depletion of Rictor decreased phosphorylation of Akt at Ser473 and PKC-α at Ser657, located in the hydrophobic domain (Fig. 5A).
Fig. 5.
Inhibition of mammalian target of rapamycin (mTOR) is not equivalent to disruption of mTOR complex 1 and/or complex 2 in affecting human aortic endothelial cell (HAEC) signaling. A and B: HAECs were transfected with siRNA and treated with or without TNF-α for 4 h, and cell lysates were analyzed by Western blot analysis for kinase phosphorylation (n = 5). C: PKC-α phosphorylation was detected and quantified in siRNA-transfected HAECs (n = 5). D: HAECs were treated with rapamycin (rapa) and torin 1 followed by stimulation with TNF-α. Cell lysates were analyzed by Western blot analysis for PKC-α phosphorylation (n = 5). 4EBP1, 4E-binding protein-1; p-, phosphorylated; p70S6K, 70-kDa ribosomal protein S6 kinase; siCtrl, control siRNA; siMTOR, mTOR-targeted siRNA; siRAP, regulatory-associated protein of mTOR (RAPTOR)-targeted siRNA; siRIC, rapamycin-insensitive companion of mTOR (RICTOR)-targeted siRNA. *P ≤ 0.05; **P ≤ 0.0001; ***P ≤ 0.0001 vs. siCtrl (one-way ANOVA followed by a Dunnett’s test).
Unexpectedly, we also consistently observed decreased PKC-α phosphorylation in Raptor-depleted HAECs (Fig. 5, A and C). Furthermore, in sharp contrast to the inhibitory effect of Raptor or Rictor depletion on PKC-α activity, MTOR depletion activated PKC-α as well as Akt (Fig. 5, B and C). Similarly, torin 1 or rapamycin treatment clearly increased PKC-α phosphorylation in both the absence and presence of TNF-α (Fig. 5D).
Even more unexpected was the differential signaling response of HAECs depleted of both Raptor and Rictor and those transfected with mTOR siRNA. Depletion of both Raptor and Rictor inhibited PKC-α phosphorylation, whereas it did not significantly affect phosphorylation of p70S6K and 4EBP1 (Fig. 5B). These results suggest that both mTORC1 and mTORC2 contributed to PKC-α activation and inhibition of mTOR is not equivalent to disruption of mTORC1 or mTORC2 singly or together in affecting downstream signaling transduction in HAECs. Specifically, mTOR suppressed PKC-α activation independent of mTORC1 and/or mTORC2 in HAECs.
PKC-α and miR-200a-3p independently modulated GATA6 expression in response to inhibition of mTOR.
Both chemical treatment (data not shown) and MTOR knockdown (Fig. 5B) inhibited phosphorylation of p70S6K and 4EBP1, which are known to affect protein translation (27). Therefore, we first investigated whether this could be a mechanism causing GATA6 downregulation. However, this was ruled out as knockdown of p70S6K or overexpression of either WT or phosphorylation site mutant (Thr37Ala and Thr46Ala) 4EBP1 did not affect GATA6 expression (data not shown). These results were consistent with those observed in Raptor-depleted HAECs, where phosphorylation of p70S6K and 4EBP1 was decreased and GATA6-mediated VCAM-1 expression was not.
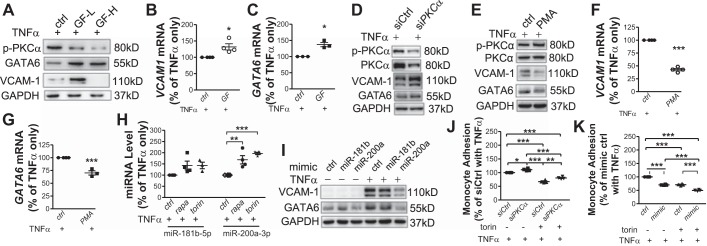
A pharmacological PKC-α inhibitor (GF109203X) was next applied to further examine the role of PKC-α in the regulatory process. At a high dose, this inhibitor of conventional PKCs completely inhibited TNF-α-induced VCAM-1 expression (Fig. 6A), indicating that a basal level of PKC-α activity is required for VCAM-1 expression as well as cell survival (data not shown). However, at the relatively low dose of 100 nM, which was sufficient to inhibit PKC-α phosphorylation (Fig. 6A) but did not affect cell viability (data not shown), GF109203X increased VCAM1 transcription (Fig. 6B) and protein levels (Fig. 6A). In our study, TNF-α transactivated GATA6 by 3.6-fold (data not shown), which was further increased by 40.3% with 100 nM GF109203X (Fig. 6C). GF109203X at either 100 nM or 10 μM increased GATA6 protein levels (Fig. 6A). Consistently, siRNA-mediated specific knockdown of PKC-α elevated TNF-α-stimulated VCAM-1 and GATA6 expression (Fig. 6D). In contrast, treatment with the PKC activator PMA attenuated TNF-α-induced VCAM1 and GATA6 transcription (Fig. 6, F and G) and protein production (Fig. 6E). Together, these results suggest a negative regulation of PKC-α on GATA6-induced VCAM1 transcription.
Fig. 6.
PKC-α and miR-200a-3p independently modulate GATA6 expression in response to inhibition of mammalian target of rapamycin. A: after 1-h pretreatment with the PKC inhibitor GF109203X at 100 nM (GF-L) or 10 μM (GF-H), human aortic endothelial cells (HAECs) were treated with TNF-α for 4 h before Western blot analysis of GATA6 and VCAM-1 expression (n = 3). B and C: HAECs were pretreated with 100 nM GF109203X (GF), stimulated with TNF-α for 2 h, and analyzed with real-time PCR for VCAM1 and GATA6 mRNA levels (n = 3–4). D: GATA6 and VCAM-1 expression in PKC-α siRNA (siPKCα)-transfected HAECs was analyzed with Western blot (n = 3). E–G: after 1-h pretreatment with the PKC activator PMA (1 μM), HAECs were incubated with TNF-α for 4 h before Western blot analysis (n = 3; E) or for 2 h before real-time PCR (n = 3–4; F and G) to measure VCAM1 and GATA6 expression. H: HAECs were pretreated with torin 1 or rapamycin (rapa) for 16 h followed by incubation with TNF-α for 2 h before quantification of each miRNA expression (n = 4). I: 48 h after transfection with control (ctrl) or each mimic miRNA, HAECs were treated with or without TNF-α for 4 h followed by Western blot analysis (n = 3). J and K: HAECs were transfected with PKC-α-targeted siRNA (J) or miR-200a-3p mimic (K) followed by treatment with or without torin 1 for 16 h. TNF-α-induced THP-1 cell adhesion (n = 4) was evaluated as described in Fig. 1, E and F. p-, phosphorylated; siCtrl, control siRNA. *P ≤ 0.05; **P ≤ 0.001; ***P ≤ 0.0001 (two-tailed paired t-test, B, C, F, and G; one-way ANOVA followed by a Newman-Keuls test, H, J, and K).
Inhibition of mTOR changed expression of multiple miRNAs (29, 41). Moreover, several miRNAs were revealed to regulate GATA6 expression mainly in cancer cells (9, 16, 38). On the basis of the literature and in silico (miRTarBase and TargetScan) analysis of the GATA6 promoter, we evaluated expression of miR-181b-5p, miR-181d-5p, miR-200a-3p, and miR-196-5p for a role in regulating GATA6 expression. In the presence of TNF-α, miR-200a-3p was significantly increased by 1.7- and 2.0-fold in rapamycin- and torin 1-treated HAECs, respectively (Fig. 6H). Both treatments also increased miR-181b-5p, although the threshold for significance was not achieved (Fig. 6H). In contrast, miR-181d-5p and miR-196-5p expression were not affected (data not shown). Experiments with a miRNA mimic confirmed the specific repression of TNF-α-induced GATA6 and VCAM-1 by miR-200a-3p and to a lesser degree by miR-181b-5p (Fig. 6I).
Inhibition or activation of PKC did not significantly affect miR-200a-3p or miR-181b-5p expression (data not shown), indicating that PKC-α and miR-200a-3p independently repressed GATA6 expression in response to inhibition of mTOR. Consistent with the effect on GATA6-mediated VCAM-1 expression, inhibition of PKC-α with siRNA reversed, whereas transfection of the miRNA-200α-3p mimic enhanced, the repressive regulation of mTOR inhibition on THP-1 adhesion onto the activated HAEC monolayer (Fig. 6, J and K).
DISCUSSION
Precise regulation of endothelial surface VCAM-1 expression is critical for normal immune function of monocytes and T cells, but dysregulation can result in the initiation and progression of atherosclerosis, which is currently recognized as a chronic inflammatory disease. mTOR signaling has been implicated in diverse physiological and pathological processes including organismal growth, cancer, type 2 diabetes, and neurodegeneration (19). However, its role in the regulation of cytokine-induced VCAM-1 expression on arterial endothelial cells as relates to atherogenesis has not been elucidated. Contrary to the canonical view of mTOR signaling, here we demonstrated that both intact mTORC1 and intact mTORC2 were required for HAEC activation of PKC-α and that inhibition of mTOR through its depletion or pharmacological inhibition enhanced PKC-α activation through a mechanism independent of disruption of mTORC1 and/or mTORC2. Activated PKC-α together with increased expression of miR-200a-3p accompanying inhibition of mTOR effectively suppressed VCAM1 transcription through downregulation of GATA6 (Fig. 7). These observations are of pathological importance in that they translated to inhibition of VCAM-1 surface expression by HAECs and concomitant decreased adhesion of monocytes. These findings elucidate the complexity of mTOR signaling in the regulation of VCAM-1 expression by arterial endothelium and challenge the conventional wisdom regarding mTOR signaling.
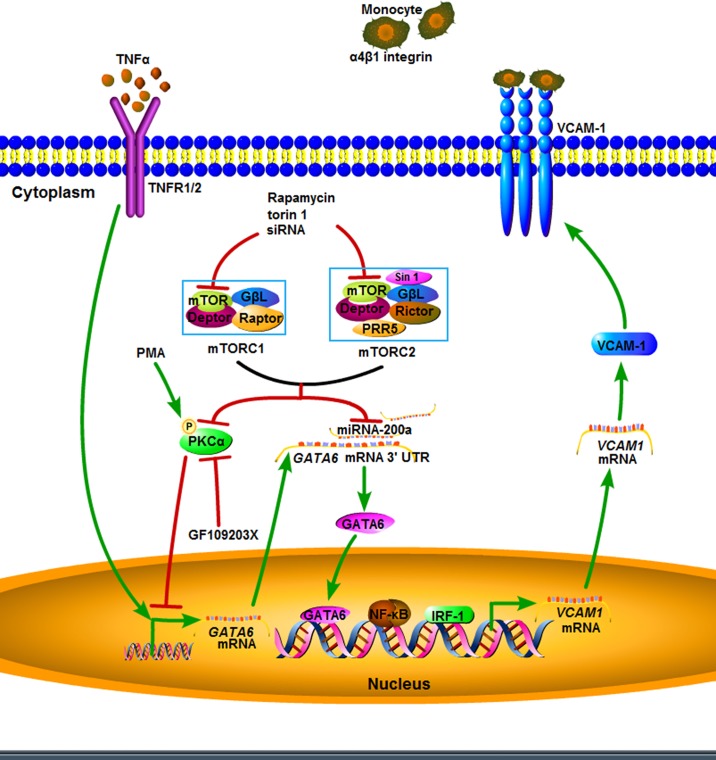
Fig. 7.
Schematic depiction of mammalian target of rapamycin (mTOR) inhibition on TNF-α-induced VCAM-1 expression on human aortic endothelial cells. Inhibition of mTOR increases the activation of PKC-α and expression of miR-200a-3p, which suppress VCAM1 transcription through downregulation of GATA6. Deptor, DEP domain-containing mTOR-interacting protein; GβL, G protein β-subunit-like (target of rapamycin complex subunit LST8); IRF-1, interferon regulatory factor 1; mTORC, mTOR complex; P, phosphorylation; PRR5, proline-rich protein-5 (protein observed with Rictor-1); Raptor, regulatory-associated protein of mTOR; Rictor, rapamycin-insensitive companion of mTOR; Sin 1, stress-activated MAPK-interacting protein-1; TNFR1/2, TNF receptor 1/2; UTR, untranslated region.
mTORC2 has been considered to be the complex responsible for regulation of PKC-α signaling and cytoskeletal organization as well as complete Akt activation (19, 30). Knockdown of mTOR or Rictor was found to inhibit PKC-α phosphorylation. In this study, an unexpected finding was that both mTORC1 and mTORC2 were required for PKC-α activation, both constitutively and in response to TNF-α stimulation. Moreover, depletion of mTOR led to increased rather than decreased PKC-α and Akt activation. This phenomenon is not attributable to either mTORC1 or mTORC2 disruption, further challenging the concept that mTOR signaling is mediated exclusively by either of the two complexes. Supporting this novel finding was that knockdown of neither Raptor nor Rictor decreased VCAM1 transcription as elicited by mTOR knockdown or rapamycin or torin 1 treatment.
A second unexpected result was that combined knockdown of Raptor and Rictor resulted in a phenotype distinct from that of mTOR depletion, reflected in p70S6K and 4EBP1 phosphorylation, PKC-α and Akt activation, and VCAM1 transcription. Activation of p70S6K leads to a feedback inhibition of Akt signaling (17). Consistent with this notion, increased Akt phosphorylation seen in mTOR-depleted HAECs might be due to the decreased p70S6K activation. The combined knockdown of Raptor or Rictor decreased PKC-α activation, further indicating that mTOR inhibited PKC-α through a mechanism independent of mTORC1 and/or mTORC2. In concert with a previous observation of an inverse correlation between the amounts of Raptor and Rictor in mTOR complexes across mammalian cell types (30), we noted changes in the stoichiometric ratio among mTOR, Raptor, and Rictor upon knockdown of any of the three components. Depletion of both Raptor and Rictor decreased mTOR expression (Fig. 2E), which was consistent with the observation in skeletal muscles isolated from combined Raptor and Rictor knockout mice (2). However, in mTOR-depleted HAECs, neither Rictor nor Raptor expression was obviously affected. One possible explanation for these results is that regulation of protein kinase AGC signaling may necessitate an appropriate stoichiometric ratio among the three components. Another possibility is that mTOR forms additional complexes beyond mTORC1 and mTORC2 that function as inhibitors of PKC-α in HAECs to tightly regulate this multifunctional kinase. Similarly, Raptor or Rictor may form other complexes to inhibit phosphorylation of p70S6K and 4EBP1 when mTOR is depleted, which may explain the observation that their phosphorylation was decreased in MTOR-deficient cells but not in HAECs depleted of both RAPTOR and RICTOR. In addition, Raptor expression was increased in control siRNA-transfected HAECs treated with TNF-α for 4 h (Fig. 2E). It is possible that TNF-α signals to upregulate Raptor expression in HAECs. These assumptions warrant further investigation.
Similar to genes encoding endothelial ICAM-1 and E-selectin, the VCAM1 gene promoter contains binding sites for NF-κB, activator protein-1, and transcription factor Sp1. Rapamycin has been reported to augment ICAM-1 expression by potentiating thrombin-induced IκBα phosphorylation and NF-κB nuclear localization (23). In another study, mTOR knockdown or rapamycin treatment was found to decrease oxidized low-density lipoprotein-induced IκBα phosphorylation and accumulation of p65 in the nucleus (40). In our study, we found that rapamycin treatment increased ICAM1 transcription in the absence of TNF-α, although NF-κB phosphorylation was not detected. In the presence of TNF-α, NF-κB phosphorylation was not significantly changed in either rapamycin-treated, torin-treated, or mTOR-depleted HAECs. These results highlight the importance of other transcriptional modulators in the cell-specific regulation of signaling pathways by mTOR.
Differences between the promoters of VCAM1 and other adhesion molecule-coding genes provided impetus to study the mechanisms underlying its specific transcriptional regulation. Compared with the ICAM1 gene, the VCAM1 promoter has a unique binding site for transcription factors IRF-1 and GATA, which play important roles in differentiating expression of VCAM1 from other adhesion molecules (8, 32, 34, 37). In our study, torin 1 or rapamycin treatment did not significantly affect TNF-α-induced ICAM1 transcription, which motivated us to examine IRF-1 and GATA6 activity. Consistent with a previous study (11), we found that rapamycin or torin 1 treatment alone or together with TNF-α significantly increased IRF-1 expression and accumulation in the nucleus and its transcriptional activity. The same study (11) reported that regulation of IRF-1 by mTOR was independent of Akt. In our study, both torin 1 and rapamycin treatment inhibited VCAM-1 expression in a time-dependent manner (data not shown). However, peak inhibition of Akt phosphorylation by torin 1 was achieved at ~1 h, and the phosphorylation was half recovered at 16 h of treatment (data not shown). Rapamycin inhibited VCAM-1 expression as early as at 4 h of treatment when Akt phosphorylation remained unchanged (data not shown). Furthermore, Akt phosphorylation was not decreased in mTOR-depleted HAECs (Fig. 5B). Similarly, in muscle cells, mTOR depletion does not affect phosphorylation of Akt at Ser473 (28). These findings contrasted with a recent report from Wang et al. (36) showing that rapamycin decreased TNF-α-induced VCAM-1 expression through inhibition of the Akt/IRF-1 cascade. This discrepancy may be due to variation in cell origin and the dose of TNF-α and rapamycin applied: notably, 10 ng/ml TNF-α and 100 ng/ml (~100 nM) rapamycin to treat human umbilical vein endothelial cells. In the present study, relatively low doses of TNF-α (0.1 ng/ml) and rapamycin (25 nM) were used to treat arterial endothelial cells without serum starvation. There is considerable evidence that mTOR signaling elicits biological responses in a tissue- and context-specific manner. For example, compared with Raptor knockout, additional knockout of Rictor abrogated the dysfunction of podocytes in kidney (12) but not the phenotype in skeletal muscle (2). Deficiency of Rictor in muscles causes no effect (2), whereas it leads to severe defects in other tissues such as kidney (12), adipose tissue (6), and the nervous system (33). Moreover, the present conditions may prove more physiologically relevant since atherosclerosis originates in arteries and serum TNF-α concentration in normal human subjects is in the picograms per milliliter range.
Among the several GATAs in endothelial cells, GATA6 is TNF-α inducible and contributes significantly to TNF-α induction of VCAM-1 (35) and differential regulation of VCAM-1 and ICAM-1 (34). Consistently, we found that TNF-α induced GATA6 transcription and protein accumulation in the nucleus. The chromatin immunoprecipitation assay confirmed that inhibition of mTOR decreased binding of GATA6 onto the VCAM1 promoter. These results suggested that elevated IRF-1 was insufficient to compensate for the decrease of GATA6 in the activation of VCAM1 transcription. It is noteworthy that the general GATA transcriptional activity measured with a luciferase reporter was not changed in rapamycin-treated cells (data not shown), suggesting that inhibition of mTOR may activate other members of the GATA family.
Studies with PKC inhibitors and activator or PKC-α-targeting siRNA clearly demonstrated a negative control of PKC-α on GATA6-mediated VCAM-1 expression. Increased activation of PKC-α at least partially contributed to the attenuation of TNF-α induction of GATA6 and VCAM-1, which was associated with mTOR inhibition. In addition, mTORC1 downstream phosphorylation of p70S6K and 4EBP1 was involved in protein translation (27). After phosphorylation, p70S6K becomes activated and phosphorylates its substrate S6 protein of the 40S ribosomal subunit, which is involved in translational regulation of mRNAs (27). 4EBP1 is a translation repressor that inhibits cap-dependent translation by binding to eukaryotic translation initiation factor 4E (eIF4E). Phosphorylation of 4EBP1 disrupts this interaction and results in the activation of cap-dependent translation (27). However, decreased expression of GATA6 was not due to inhibition of p70S6K or low-level phosphorylation of 4EBP1 caused by mTOR knockdown or rapamycin or torin 1 treatment.
miRNAs are involved in maintaining endothelial function (10, 32) and the development of atherosclerosis (25). In addition to controlling translation, mTOR signaling regulates expression of a multitude of miRNAs leading to modification of cellular processes (29, 41). On the basis of miRNA profiling studies, in silico prediction, and previous experimental evidence (9, 16, 38), we investigated the effect of mTOR inhibition on expression of miR-181b-5p, miR-181d-5p, miR-200a-3p, and miR-196-5p. Although each of these has sequences that are complementary to the 3′-untranslated region of GATA6 mRNA, only miR-200a-3p was significantly upregulated by rapamycin or torin 1 and in turn inhibited expression of TNF-α-induced GATA6 and VCAM-1 expression. In addition to GATA6, miR-200a directly targets VCAM-1 (15), which may be another mechanism of rapamycin and torin 1 inhibition on VCAM-1 expression. In another study, miR-200a inhibited GATA6 expression and was involved in regulation of the p110α catalytic subunit of phosphatidylinositol 3-kinase and thus tumor cell metastasis (38). However, to date, how miR-200a-3p expression itself is regulated remains ill defined. In this study, we did not observe a cause-effect relationship between PKC-α activation and miR-200a-3p expression, although both contributed to GATA6 repression. Further investigation is warranted to search for the factors that are modulated by mTOR signaling and could interact with the regulatory element of the miR-200a gene that is located on human chromosome 1.
Herein, we provide novel insights into the molecular regulation of mTOR signaling in eliciting cytokine-stimulated VCAM-1 expression on the arterial endothelium, through transcriptional and posttranscriptional mechanisms regulating GATA6 expression. Further detailed understanding of the dynamics and molecular anatomy associated with precise regulation of VCAM-1 may facilitate the discovery of effective interventions targeting the inflammatory origins of atherosclerosis.
GRANTS
This work was supported by National Natural Science Foundation of China Grants 81870355 and 81670410 (to C. Sun), Natural Science Foundation of the Jiangsu Higher Education Institutions of China Grant 18KJA310001 (to C. Sun), and National Heart, Lung, and Blood Institute Grant HL-082689 (to S. I. Simon and A. G. Passerini).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.S. conceived and designed research; X.F., X.C., Q.F., K.P., Q.W., and C.S. performed experiments; X.F., X.C., Q.F., K.P., Q.W., and C.S. analyzed data; X.F., X.C., Q.F., A.G.P., S.I.S., and C.S. interpreted results of experiments; X.F., X.C., K.P., Q.W., and C.S. prepared figures; C.S. drafted manuscript; A.G.P., S.I.S., and C.S. edited and revised manuscript; X.F., X.C., Q.F., K.P., Q.W., A.G.P., S.I.S., and C.S. approved final version of manuscript.
REFERENCES
- 1.Baetta R, Granata A, Canavesi M, Ferri N, Arnaboldi L, Bellosta S, Pfister P, Corsini A. Everolimus inhibits monocyte/macrophage migration in vitro and their accumulation in carotid lesions of cholesterol-fed rabbits. J Pharmacol Exp Ther 328: 419–425, 2009. doi: 10.1124/jpet.108.144147. [DOI] [PubMed] [Google Scholar]
- 2.Bentzinger CF, Romanino K, Cloëtta D, Lin S, Mascarenhas JB, Oliveri F, Xia J, Casanova E, Costa CF, Brink M, Zorzato F, Hall MN, Rüegg MA. Skeletal muscle-specific ablation of raptor, but not of rictor, causes metabolic changes and results in muscle dystrophy. Cell Metab 8: 411–424, 2008. doi: 10.1016/j.cmet.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Brånén L, Hovgaard L, Nitulescu M, Bengtsson E, Nilsson J, Jovinge S. Inhibition of tumor necrosis factor-α reduces atherosclerosis in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol 24: 2137–2142, 2004. doi: 10.1161/01.ATV.0000143933.20616.1b. [DOI] [PubMed] [Google Scholar]
- 4.Cahill PA, Redmond EM. Vascular endothelium: gatekeeper of vessel health. Atherosclerosis 248: 97–109, 2016. doi: 10.1016/j.atherosclerosis.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castro C, Campistol JM, Sancho D, Sánchez-Madrid F, Casals E, Andrés V. Rapamycin attenuates atherosclerosis induced by dietary cholesterol in apolipoprotein-deficient mice through a p27Kip1-independent pathway. Atherosclerosis 172: 31–38, 2004. doi: 10.1016/j.atherosclerosis.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Cybulski N, Polak P, Auwerx J, Rüegg MA, Hall MN. mTOR complex 2 in adipose tissue negatively controls whole-body growth. Proc Natl Acad Sci USA 106: 9902–9907, 2009. doi: 10.1073/pnas.0811321106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cybulsky MI, Iiyama K, Li H, Zhu S, Chen M, Iiyama M, Davis V, Gutierrez-Ramos JC, Connelly PW, Milstone DS. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J Clin Invest 107: 1255–1262, 2001. doi: 10.1172/JCI11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dagia NM, Harii N, Meli AE, Sun X, Lewis CJ, Kohn LD, Goetz DJ. Phenyl methimazole inhibits TNF-α-induced VCAM-1 expression in an IFN regulatory factor-1-dependent manner and reduces monocytic cell adhesion to endothelial cells. J Immunol 173: 2041–2049, 2004. doi: 10.4049/jimmunol.173.3.2041. [DOI] [PubMed] [Google Scholar]
- 9.Fantini S, Salsi V, Reggiani L, Maiorana A, Zappavigna V. The miR-196b miRNA inhibits the GATA6 intestinal transcription factor and is upregulated in colon cancer patients. Oncotarget 8: 4747–4759, 2017. doi: 10.18632/oncotarget.13580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernández-Hernando C, Suárez Y. MicroRNAs in endothelial cell homeostasis and vascular disease. Curr Opin Hematol 25: 227–236, 2018. doi: 10.1097/MOH.0000000000000424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fielhaber JA, Han YS, Tan J, Xing S, Biggs CM, Joung KB, Kristof AS. Inactivation of mammalian target of rapamycin increases STAT1 nuclear content and transcriptional activity in α4- and protein phosphatase 2A-dependent fashion. J Biol Chem 284: 24341–24353, 2009. doi: 10.1074/jbc.M109.033530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gödel M, Hartleben B, Herbach N, Liu S, Zschiedrich S, Lu S, Debreczeni-Mór A, Lindenmeyer MT, Rastaldi MP, Hartleben G, Wiech T, Fornoni A, Nelson RG, Kretzler M, Wanke R, Pavenstädt H, Kerjaschki D, Cohen CD, Hall MN, Rüegg MA, Inoki K, Walz G, Huber TB. Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. J Clin Invest 121: 2197–2209, 2011. doi: 10.1172/JCI44774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iiyama K, Hajra L, Iiyama M, Li H, DiChiara M, Medoff BD, Cybulsky MI. Patterns of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 expression in rabbit and mouse atherosclerotic lesions and at sites predisposed to lesion formation. Circ Res 85: 199–207, 1999. doi: 10.1161/01.RES.85.2.199. [DOI] [PubMed] [Google Scholar]
- 14.Ikenoue T, Inoki K, Yang Q, Zhou X, Guan KL. Essential function of TORC2 in PKC and Akt turn motif phosphorylation, maturation and signalling. EMBO J 27: 1919–1931, 2008. doi: 10.1038/emboj.2008.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iorio MV, Visone R, Di Leva G, Donati V, Petrocca F, Casalini P, Taccioli C, Volinia S, Liu CG, Alder H, Calin GA, Ménard S, Croce CM. MicroRNA signatures in human ovarian cancer. Cancer Res 67: 8699–8707, 2007. doi: 10.1158/0008-5472.CAN-07-1936. [DOI] [PubMed] [Google Scholar]
- 16.Ji J, Yamashita T, Budhu A, Forgues M, Jia HL, Li C, Deng C, Wauthier E, Reid LM, Ye QH, Qin LX, Yang W, Wang HY, Tang ZY, Croce CM, Wang XW. Identification of microRNA-181 by genome-wide screening as a critical player in EpCAM-positive hepatic cancer stem cells. Hepatology 50: 472–480, 2009. doi: 10.1002/hep.22989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Julien LA, Carriere A, Moreau J, Roux PP. mTORC1-activated S6K1 phosphorylates Rictor on threonine 1135 and regulates mTORC2 signaling. Mol Cell Biol 30: 908–921, 2010. doi: 10.1128/MCB.00601-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karmann K, Min W, Fanslow WC, Pober JS. Activation and homologous desensitization of human endothelial cells by CD40 ligand, tumor necrosis factor, and interleukin 1. J Exp Med 184: 173–182, 1996. doi: 10.1084/jem.184.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell 149: 274–293, 2012. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee DY, Lin TE, Lee CI, Zhou J, Huang YH, Lee PL, Shih YT, Chien S, Chiu JJ. MicroRNA-10a is crucial for endothelial response to different flow patterns via interaction of retinoid acid receptors and histone deacetylases. Proc Natl Acad Sci USA 114: 2072–2077, 2017. [Erratum in Proc Natl Acad Sci USA 114: E2985, 2017.] doi: 10.1073/pnas.1621425114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol 7: 678–689, 2007. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 22.Liu Q, Kang SA, Thoreen CC, Hur W, Wang J, Chang JW, Markhard A, Zhang J, Sim T, Sabatini DM, Gray NS. Development of ATP-competitive mTOR inhibitors. Methods Mol Biol 821: 447–460, 2012. doi: 10.1007/978-1-61779-430-8_29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Minhajuddin M, Fazal F, Bijli KM, Amin MR, Rahman A. Inhibition of mammalian target of rapamycin potentiates thrombin-induced intercellular adhesion molecule-1 expression by accelerating and stabilizing NF-κB activation in endothelial cells. J Immunol 174: 5823–5829, 2005. doi: 10.4049/jimmunol.174.9.5823. [DOI] [PubMed] [Google Scholar]
- 24.Mueller MA, Beutner F, Teupser D, Ceglarek U, Thiery J. Prevention of atherosclerosis by the mTOR inhibitor everolimus in LDLR−/− mice despite severe hypercholesterolemia. Atherosclerosis 198: 39–48, 2008. doi: 10.1016/j.atherosclerosis.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 25.Novák J, Olejníčková V, Tkáčová N, Santulli G. Mechanistic role of microRNAs in coupling lipid metabolism and atherosclerosis. Adv Exp Med Biol 887: 79–100, 2015. doi: 10.1007/978-3-319-22380-3_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oshiro N, Yoshino K, Hidayat S, Tokunaga C, Hara K, Eguchi S, Avruch J, Yonezawa K. Dissociation of raptor from mTOR is a mechanism of rapamycin-induced inhibition of mTOR function. Genes Cells 9: 359–366, 2004. doi: 10.1111/j.1356-9597.2004.00727.x. [DOI] [PubMed] [Google Scholar]
- 27.Richter JD, Sonenberg N. Regulation of cap-dependent translation by eIF4E inhibitory proteins. Nature 433: 477–480, 2005. doi: 10.1038/nature03205. [DOI] [PubMed] [Google Scholar]
- 28.Risson V, Mazelin L, Roceri M, Sanchez H, Moncollin V, Corneloup C, Richard-Bulteau H, Vignaud A, Baas D, Defour A, Freyssenet D, Tanti JF, Le-Marchand-Brustel Y, Ferrier B, Conjard-Duplany A, Romanino K, Bauché S, Hantaï D, Mueller M, Kozma SC, Thomas G, Rüegg MA, Ferry A, Pende M, Bigard X, Koulmann N, Schaeffer L, Gangloff YG. Muscle inactivation of mTOR causes metabolic and dystrophin defects leading to severe myopathy. J Cell Biol 187: 859–874, 2009. doi: 10.1083/jcb.200903131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santulli G, Totary-Jain H. Tailoring mTOR-based therapy: molecular evidence and clinical challenges. Pharmacogenomics 14: 1517–1526, 2013. doi: 10.2217/pgs.13.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol 14: 1296–1302, 2004. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 31.Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell 22: 159–168, 2006. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 32.Sun C, Alkhoury K, Wang YI, Foster GA, Radecke CE, Tam K, Edwards CM, Facciotti MT, Armstrong EJ, Knowlton AA, Newman JW, Passerini AG, Simon SI. IRF-1 and miRNA126 modulate VCAM-1 expression in response to a high-fat meal. Circ Res 111: 1054–1064, 2012. doi: 10.1161/CIRCRESAHA.112.270314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomanetz V, Angliker N, Cloëtta D, Lustenberger RM, Schweighauser M, Oliveri F, Suzuki N, Rüegg MA. Ablation of the mTORC2 component rictor in brain or Purkinje cells affects size and neuron morphology. J Cell Biol 201: 293–308, 2013. doi: 10.1083/jcb.201205030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsoyi K, Jang HJ, Nizamutdinova IT, Park K, Kim YM, Kim HJ, Seo HG, Lee JH, Chang KC. PTEN differentially regulates expressions of ICAM-1 and VCAM-1 through PI3K/Akt/GSK-3β/GATA-6 signaling pathways in TNF-α-activated human endothelial cells. Atherosclerosis 213: 115–121, 2010. doi: 10.1016/j.atherosclerosis.2010.07.061. [DOI] [PubMed] [Google Scholar]
- 35.Umetani M, Mataki C, Minegishi N, Yamamoto M, Hamakubo T, Kodama T. Function of GATA transcription factors in induction of endothelial vascular cell adhesion molecule-1 by tumor necrosis factor-α. Arterioscler Thromb Vasc Biol 21: 917–922, 2001. doi: 10.1161/01.ATV.21.6.917. [DOI] [PubMed] [Google Scholar]
- 36.Wang C, Qin L, Manes TD, Kirkiles-Smith NC, Tellides G, Pober JS. Rapamycin antagonizes TNF induction of VCAM-1 on endothelial cells by inhibiting mTORC2. J Exp Med 211: 395–404, 2014. doi: 10.1084/jem.20131125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Warfel JM, D’Agnillo F. Anthrax lethal toxin enhances TNF-induced endothelial VCAM-1 expression via an IFN regulatory factor-1-dependent mechanism. J Immunol 180: 7516–7524, 2008. doi: 10.4049/jimmunol.180.11.7516. [DOI] [PubMed] [Google Scholar]
- 38.Yang Y, Ahn YH, Chen Y, Tan X, Guo L, Gibbons DL, Ungewiss C, Peng DH, Liu X, Lin SH, Thilaganathan N, Wistuba II, Rodriguez-Canales J, McLendon G, Creighton CJ, Kurie JM. ZEB1 sensitizes lung adenocarcinoma to metastasis suppression by PI3K antagonism. J Clin Invest 124: 2696–2708, 2014. doi: 10.1172/JCI72171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu K, Shi C, Toral-Barza L, Lucas J, Shor B, Kim JE, Zhang WG, Mahoney R, Gaydos C, Tardio L, Kim SK, Conant R, Curran K, Kaplan J, Verheijen J, Ayral-Kaloustian S, Mansour TS, Abraham RT, Zask A, Gibbons JJ. Beyond rapalog therapy: preclinical pharmacology and antitumor activity of WYE-125132, an ATP-competitive and specific inhibitor of mTORC1 and mTORC2. Cancer Res 70: 621–631, 2010. doi: 10.1158/0008-5472.CAN-09-2340. [DOI] [PubMed] [Google Scholar]
- 40.Zhou YD, Cao XQ, Liu ZH, Cao YJ, Liu CF, Zhang YL, Xie Y. Rapamycin inhibits oxidized low density lipoprotein uptake in human umbilical vein endothelial cells via mTOR/NF-κB/LOX-1 pathway. PLoS One 11: e0146777, 2016. doi: 10.1371/journal.pone.0146777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zou Z, Chen J, Liu A, Zhou X, Song Q, Jia C, Chen Z, Lin J, Yang C, Li M, Jiang Y, Bai X. mTORC2 promotes cell survival through c-Myc-dependent up-regulation of E2F1. J Cell Biol 211: 105–122, 2015. doi: 10.1083/jcb.201411128. [DOI] [PMC free article] [PubMed] [Google Scholar]