Abstract
Diets high in salt can lead to endothelial dysfunction, a nontraditional risk factor for cardiovascular disease (CVD). Exercise is known to reduce CVD risk; however, it remains unknown whether chronic physical activity can attenuate salt-induced endothelial dysfunction independent of blood pressure (BP) and whether these changes are due to an upregulation in endogenous antioxidants. Eight-week-old Sprague-Dawley rats were fed either a normal (NS; 0.49%)- or a high (HS; 4.0%)-salt diet and further divided into voluntary wheel running (NS-VWR, HS-VWR) and sedentary (NS, HS) groups for 6 wk. BP was measured weekly and remained unchanged within groups (P = 0.373). Endothelium-dependent relaxation (EDR) was impaired in the femoral artery of HS compared with NS (38.6 ± 4.0% vs. 65.0 ± 3.6%; P = 0.013) animals, whereas it was not different between NS and HS-VWR (73.4 ± 6.4%; P = 0.273) animals. Incubation with the antioxidants TEMPOL (P = 0.024) and apocynin (P = 0.013) improved EDR in HS animals, indicating a role for reactive oxygen species (ROS). Wheel running upregulated the antioxidant superoxide dismutase-2 (SOD-2) (P = 0.011) under HS conditions and lowered NOX4 and Gp91-phox, two subunits of NADPH oxidase. Wheel running elevated phosphorylated endothelial nitric oxide synthase (eNOS) (P = 0.014) in HS-fed rats, demonstrating a role for physical activity and eNOS activity under HS conditions. Finally, there was a reduction in EDR (P = 0.038) when femoral arteries from NS-VWR animals were incubated with TEMPOL or apocynin, suggesting there may be a critical level of ROS needed to maintain endothelial function. In summary, physical activity protected HS-fed rats from reductions in endothelial function, likely through increased SOD-2 levels and reduced oxidative stress.
NEW & NOTEWORTHY Our data suggest that voluntary wheel running can prevent impairments in endothelium-dependent relaxation in the femoral artery of rats fed a high-salt diet. This appears to be independent of blood pressure and mediated through a decrease in expression of NADPH oxidases as a result of physical activity. These data suggest that increased chronic physical activity can protect the vasculature from a diet high in salt, likely through a reduction in oxidative stress.
Keywords: endothelium, exercise, sodium
INTRODUCTION
A diet high in salt leads to an increased risk of cardiovascular disease (CVD) (1, 30). Endothelial dysfunction is an initial event in the development of atherosclerosis and is associated with increased CVD risk (21, 24). Recent work has shown that salt has a harmful effect on the vasculature before and independent of changes in blood pressure (BP) (8, 11, 16, 18). Nitric oxide (NO), an important vasodilator, is responsible for the maintenance of a healthy vasculature (10, 15, 33). A loss in the bioavailability and/or production of NO disrupts vascular homeostasis and can result in endothelial dysfunction (13). High-salt (HS) diets have been implicated in reducing NO bioavailability (16, 28).
Although the mechanism for salt-induced damage in the vasculature is not completely understood, it has been linked to increases in reactive oxygen species (ROS) (16, 17), specifically superoxide (O2·−) (29, 39, 40) and the membrane-associated enzymes NADPH oxidases (NOXs) (16, 39, 40). Increased levels of ROS are known to decrease NO bioavailability/production by oxidizing nitric oxide synthase (NOS) or critical cofactors essential for NO synthesis and/or by reacting directly with NO and therefore forming potent oxidants (23). This ultimately leads to reduced endothelial function (17, 29, 39).
Regular aerobic exercise has been demonstrated to reduce CVD risk in both humans (2, 12) and rodent models (9, 19, 25). Furthermore, both voluntary (9, 19) and forced (32, 37) aerobic exercise have been shown to attenuate endothelial dysfunction over 4–14 wk in several rodent models including aging (9), a western-style diet (19), chronic kidney disease (25), and spontaneous hypertension (36). One mechanism responsible for this attenuation is thought to be the upregulation of endogenous antioxidants (9, 19, 32, 37). This exercise-mediated rise in antioxidants, in particular superoxide dismutase (SOD), was shown to prevent a ROS-induced decline in NO bioavailability (32, 35, 38). However, it is not known whether chronic wheel running can attenuate the effects of salt on the vasculature before elevations in BP and subsequent CVD.
In the present study, we tested the hypothesis that voluntary wheel running (VWR) would preserve endothelial function in rats fed a HS diet independent of changes in BP. Additionally, we hypothesized that wheel running would reduce oxidative stress and preserve NO in the femoral artery of rats fed a HS diet. To test these hypotheses, we used VWR as our model of physical activity. During the 6-wk intervention, animals consumed either a normal-salt (NS) or a HS diet. Endothelial function, BP, antioxidant status, and oxidative damage were assessed at the end of 6 wk.
MATERIALS AND METHODS
Ethical approval.
All surgical procedures and experiments were approved by the University of Delaware Institutional Animal Care and Use Committee and were conducted in accordance with the Public Health Service Policy on Humane Care and Use of Laboratory Animals.
Animal model.
Sixty 8-wk-old male Sprague-Dawley rats (Harlan Laboratories) were housed under standard conditions at the University of Delaware Animal Facility. This included ad libitum access to food and water and a 12:12-h light-dark cycle. Animals were acclimatized to the facility for 1 wk before random stratification into two diet groups: NS or HS. The NS diet group was fed a 0.49% NaCl diet and the HS diet group a 4.0% NaCl rat chow (NS: TD.96208, HS: TD.92034; Harlan Teklad). A 4.0% NaCl diet has been previously shown to result in endothelial dysfunction in a rodent model (39, 40). Body mass was measured weekly to determine whether any changes in body mass occurred because of the dietary intervention.
The two diet groups were further subdivided into a VWR group and a sedentary group, resulting in four groups: NS, HS, NS-VWR, and HS-VWR. The VWR groups were placed in a cage with a running wheel attached, giving them free access to run at will. Wheel revolutions were recorded daily and used with wheel diameter to calculate total distance run. Total distance run was calculated weekly. Six weeks after the start of the diet and/or wheel running, animals were removed from their cage 16–24 h before euthanasia. Animals were euthanized, and one femoral artery was used immediately to measure endothelium-dependent relaxation (EDR) and the other frozen for tissue analysis. Aortic segments and the soleus muscle were also snap frozen and stored at −80°C for tissue analysis.
Measurements of sodium excretion and serum sodium concentration.
Urinary and serum electrolyte levels (Easy Electrolyte Analyzer; Medica, Bedford, MA) were assessed at baseline and at 6 wk. Animals were placed in metabolic cages for 16 h to collect their urine. During this time period they only had access to water, to avoid food mixing with the urine. Urine was collected in a urine collection cylinder at the bottom of the cage. Urine volume was measured, divided into aliquots, and used to measure urinary levels of sodium. The remainder was stored at −80°C. Electrolytes were expressed as millimoles per 24 h per body weight × 100 g. Blood samples were taken via a tail vein at baseline while the rats were anesthetized under 5.0% isoflurane and maintained at 1.5–2.5% isoflurane. Six-week blood samples were taken from the vena cava immediately before euthanasia. Blood samples were used to assess serum sodium concentrations. All remaining serum was stored at −80°C for later use.
Blood pressure measurements.
BP was measured weekly by tail cuff plethysmyography (BP-2000 Series II; Visitech Systems, Physiological Research Instruments) during the animal’s light cycle (7:00 AM to 10:00 AM). Starting 1 wk before the dietary intervention, animals were familiarized with the procedure. The cuff was placed on the animals’ tail daily to prevent stress-induced fluctuations in BP. Measurements were taken three times a week for the following 6 weeks. The average of 10 BP measurements was taken after 10 familiarization measurements to establish a daily average. A weekly mean arterial pressure (MAP) value was then calculated for each animal.
Isometric ring studies.
Vascular relaxation was assessed ex vivo by performing isometric ring studies in conductance vessel segments. Animals were anesthetized with 5% isoflurane. The chest was cut open, and animals were euthanized by exsanguination. The femoral artery was dissected and placed in an ice-cold physiological salt solution (PSS; in mmol: 118.99 NaCl, 4.69 KCl, 2.50 CaCl2·2H2O, 1.17 MgSO4·7H2O, 1.18 KH2PO4, and 0.03 EDTA, with 1.091 g/l glucose and 2.100 g/l NaHCO3; pH 7.4). Femoral artery tissue was cleaned, cut into 2-mm sections, and mounted on a 200-μm wire myograph (DMT 610M; Danish Myotechnology) for assessment of vascular responses to vasoconstricting and vasorelaxating substances. Each femoral vessel was mounted within an individual organ bath containing PSS at 37°C. Vessels were oxygenated with carbogen gas (5% of CO2-95% of O2), and resting tension was set after normalization of the length-tension curve. Femoral rings underwent a 1-h period of equilibration followed by an assessment of vessel viability. This involved constricting the rings with phenylephrine (3 × 10−7 M) and relaxing them with a single dose of acetylcholine (ACh; 10−4 M) as previously reported (22).
After the initial assessment of vessel viability, rings were washed every 10 min with PSS for an hour. Rings were then preconstricted with a single dose of phenylephrine (3 × 10−7 M) and relaxed with ACh (10−9 to 10−5 M) back to resting tension. Experiments were repeated in the presence of the NOS inhibitor NG-nitro-l-arginine methyl ester (l-NAME, 10 μM; Sigma-Aldrich catalog no. 51298-62-5) to determine the NO-mediated portion of relaxation. Femoral rings were then relaxed with ACh in a concentration-dependent manner in the presence of 30 μM TEMPOL (SOD mimetic; Sigma-Aldrich catalog no. 176141), 30 μM apocynin (APO, NADPH oxidase inhibitor; Sigma-Aldrich catalog no. 498-02-2), or TEMPOL + APO (30 μM TEMPOL + 30 μM APO) combined. Finally, after a final washout period, femoral rings were relaxed with sodium nitroprusside (SNP, 10−9 to 10−5 M) in a concentration-dependent manner to assess endothelium-independent relaxation (EIR) reflecting smooth muscle function. Concentration-response curves were fit with nonlinear regression, and the logEC50 was calculated as the concentration of the agonist needed to elicit a 50% response. Maximal relaxation (Emax) was calculated to determine the maximal relaxation achieved with each concentration-response curve.
Tissue analysis.
Femoral and aortic tissue was homogenized in a lysis buffer (in mM: 150 NaCl, 50 Tris·HCl, pH 8.0) containing 1.0% Triton X-100 (Sigma-Aldrich catalog no. 9002-93-1), protease (Sigma-Aldrich catalog no. P8340), and phosphatase inhibitors (Sigma-Aldrich catalog no. P0044). All tissue was homogenized with a Bullet Blender (Next Advance). Tissue homogenates were then centrifuged at 10,000 g for 10 min at 4°C, and the supernatant was extracted for analysis. Protein concentration was assessed with a Pierce BCA Protein Assay Kit (Thermo Scientific catalog no. 23227). Samples were loaded onto 10% Tris·HCl gels (Thermo Scientific), transferred to nitrocellulose membranes, and stained with primary antibodies (all purchased from Santa Cruz Biotechnology) for the specific protein of interest: endothelial NOS (eNOS, 1:1,000; sc-376751), phosphorylated eNOS (ser1177, 1:1,000; sc-293032), nitrotyrosine (1:1,000; sc-32757), SOD-1 (1:1,000; sc-271014), SOD-2 (1:1,000; sc-137254), catalase (1:500; sc-271803), glutathione peroxidase-1/2 (GPx-1/2, 1:500; sc-133152), NADPH oxidase 4 (Nox4, 1:500; sc-55142), and NADPH oxidase 2/Gp91-phox (1:500; sc-74514). Membranes were then reacted with a rabbit (sc-358920) or goat (sc-2031) polyclonal secondary antibody to mouse IgG. Membranes were incubated for 5 min in a substrate working solution containing Luminol Enhancer Solution (WesternSure catalog no. 926-80020) and Stable Peroxide Solution (WesternSure catalog no. 926-80020) and scanned on a C-DiGit Blot Scanner (LI-COR CDG-002561). Protein abundance was expressed as relative units normalized to β-actin (Santa Cruz Biotechnology; sc-81178) and quantified with Image Digits Studio (version. 5.0). Because of the limited amount of femoral artery tissue available, aortic tissue was used as a surrogate to perform a subset of Western blotting experiments.
Citrate synthase activity.
Soleus muscle tissue was prepared as previously described for femoral and aortic samples. Protein concentration was assessed with a Pierce BCA Protein Assay Kit (Thermo Scientific catalog no. 23227). Citrate synthase (CS) activity was measured with a CS activity kit (Sigma-Aldrich catalog no. CS0720).
Statistical analysis.
A one-way analysis of variance (ANOVA) was performed for body weight, serum and urinary electrolytes, wheel revolutions, CS activity, BP, logEC50, area under the curve (AUC), and Emax of the concentration-response curves and for femoral and aortic tissue analysis by Western blot. When a significant interaction was found, Tukey’s post hoc testing was performed. A two-way repeated-measures ANOVA with a Tukey’s post hoc test was used for concentration-response curves that included the exogenous antioxidants TEMPOL and APO. Pearson correlations were performed to examine relationships between AUC and Western blot protein expression. Concentration-response curves were generated for all vascular function data with GraphPad Prism 5.0 software and normalized to percent relaxation from each individual vessel phenylephrine (3 × 10−7 M) constriction value, which was set to 0%. Concentration-response curves were fit with a nonlinear regression, and Emax, AUC, and logEC50 were calculated. LogEC50 was calculated as the concentration of the agonist needed to elicit a 50% response. The α level was set at P < 0.05, and all data are presented as means ± SE.
RESULTS
Animal characteristics.
Animal characteristics for all four groups including body mass, heart mass, serum sodium, urinary sodium excretion, and MAP can be found in Table 1. Body mass was significantly increased over the 6 wk compared with baseline across all groups, whereas there was no difference in heart mass between groups. At the 6-wk time point, both HS and HS-VWR groups had a significant increase in 24-h urinary sodium excretion compared with baseline. Serum sodium rose in all groups at 6 wk. This was attributed to the difference in salt content between the NS diet and standard chow. Finally, MAP remained unchanged throughout the 6-wk study in all groups.
Table 1.
Animal characteristics
| NS (n = 12) |
NS-VWR (n = 13) |
HS (n = 14) |
HS-VWR (n = 12) |
|||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Week 6 | Baseline | Week 6 | Baseline | Week 6 | Baseline | Week 6 | |
| Body mass, g | 271 ± 6 | 382 ± 10* | 275 ± 7.8 | 377 ± 10* | 274 ± 7.8 | 384 ± 7* | 279 ± 6.5 | 377 ± 6.4* |
| Heart mass, g/BW | 0.36 ± 0.01 | 0.39 ± 0.02 | 0.36 ± 0.01 | 0.35 ± 0.01 | ||||
| Serum sodium, mmol/l | 136 ± 0.7 | 139 ± 0.6* | 135 ± 0.8 | 139 ± 0.4* | 138 ± 0.6 | 140 ± 0.4* | 138 ± 0.5 | 140 ± 0.5* |
| Urinary sodium excretion, mmol·l−1·24 h−1 | 388 ± 57 | 457 ± 66 | 396 ± 68 | 610 ± 90 | 419 ± 66 | 1,897 ± 362*†‡ | 547 ± 79 | 1,813 ± 283*†‡ |
| MAP, mmHg | 103 ± 4 | 105 ± 3 | 107 ± 3 | 106 ± 3 | 106 ± 3 | 108 ± 3 | 106 ± 3 | 108 ± 3 |
Values are means ± SE for n animals. BW, body weight; HS, high salt; MAP, mean arterial pressure; NS, normal salt; VWR, voluntary wheel running.
vs. baseline,
vs. NS 6 wk,
vs. NS-VWR 6 wk (P < 0.05).
Running distance was not different between wheel running groups, nor was there an effect of time. The NS-VWR group ran a weekly average of 10,884 ± 4,119 m, whereas the HS-VWR group ran 14,692 ± 3,760 m. CS activity was measured to determine the efficacy of the VWR perturbation. Average CS activity was significantly elevated in the wheel running groups (NS-VWR 666 ± 84 µm·ml−1·min−1, HS-VWR 809 ± 151 µm·ml−1·min−1) compared with the sedentary groups (NS 324 ± 49 µm·ml−1·min−1, HS 302 ± 63 µm·ml−1·min−1; P = 0.013). There was no effect of diet on CS activity.
Effect of salt and wheel running on endothelial function.
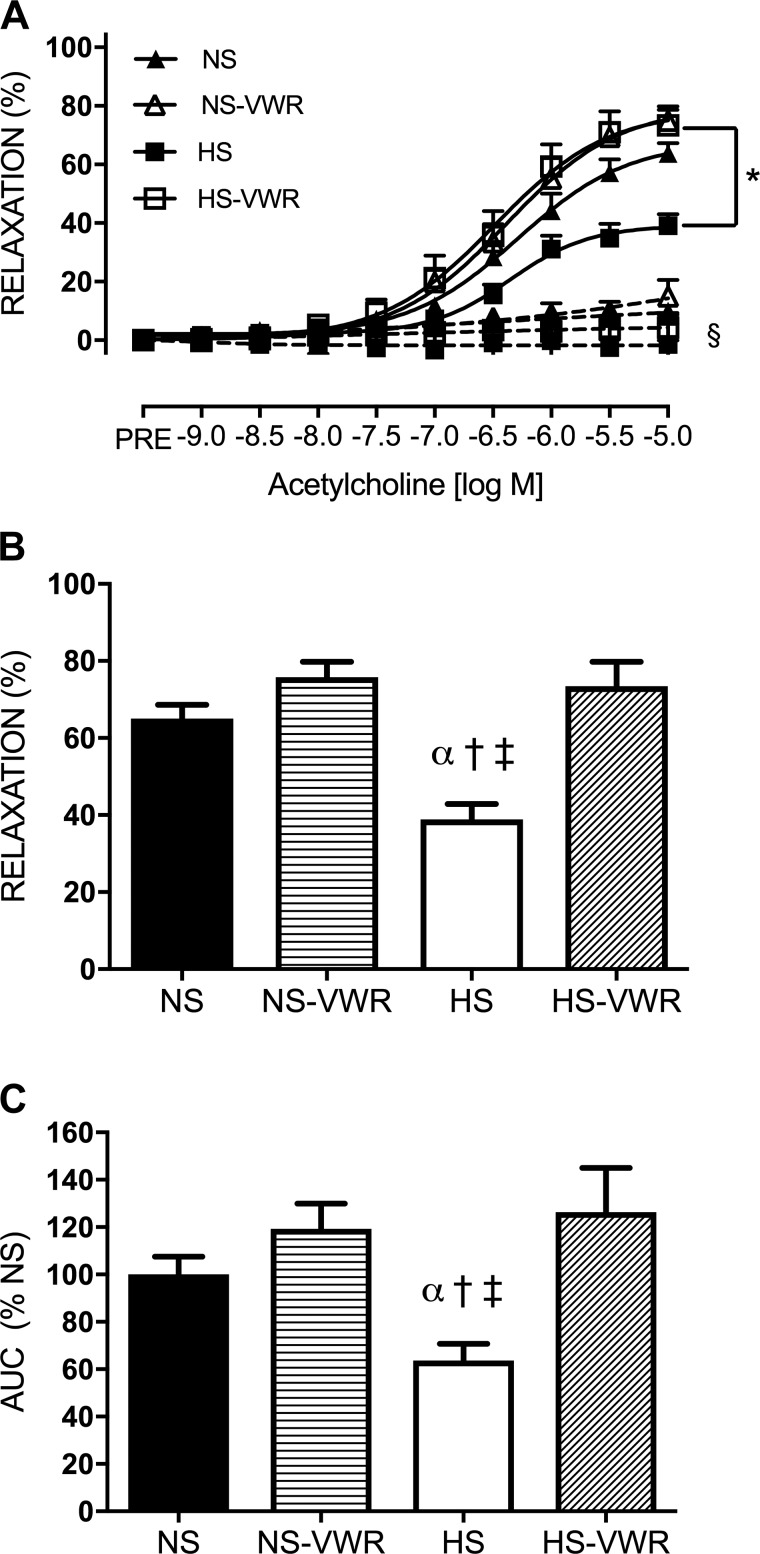
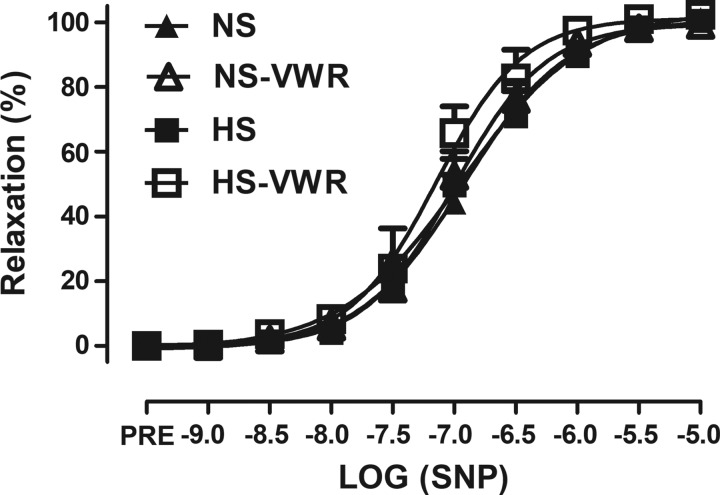
There were no differences in femoral ring resting tension between the groups (NS 4.1 ± 0.05 μN, NS-VWR 4.6 ± 0.7 μN, HS 4.1 ± 0.4 μN, HS-VWR 4.2 ± 0.4 μN; P = 0.338). The response to increasing concentrations of ACh in the femoral artery is shown in Fig. 1. EDR was impaired in the HS group compared with all other groups (Fig. 1A) as well as Emax (Fig. 1B). Specifically, after 6 wk of high salt, Emax was 26% lower in the HS group than the NS group. The HS-VWR group demonstrated greater maximal relaxation and was not different from NS or NS-VWR. AUC was significantly higher in the NS, NS-VWR, and HS-VWR groups compared with HS (Fig. 1C). Administration of l-NAME reduced relaxation in all groups compared with their respective controls (Fig. 1A; P < 0.0001). Finally, there were no group differences in response to SNP (Fig. 2).
Fig. 1.
Endothelium-dependent relaxation (EDR) in sedentary and voluntary wheel running (VWR) rats fed either a normal-salt (NS) or a high-salt (HS) diet: dose responses of femoral rings to acetylcholine (ACh) under control (solid lines) and NG-nitro-l-arginine methyl ester (l-NAME)-treated (dashed lines) conditions (A), maximal relaxation (Emax) to ACh (B), and area under the curve (AUC) to ACh (C). Values are means ± SE; n: NS = 12, NS-VWR = 13, HS = 14, HS-VWR = 12. Data analysis was performed with a 1-way ANOVA with a Tukey’s post hoc test. *vs. HS, α vs. NS, †vs. NS-VWR, ‡vs. HS-VWR, §vs. respective control group (P < 0.05).
Fig. 2.
Endothelium-independent relaxation (EIR) in sedentary and voluntary wheel running (VWR) rats fed either a normal-salt (NS) or a high-salt (HS) diet. Values are means ± SE; n: NS = 12, NS-VWR = 13, HS = 14, HS-VWR = 12. Data analysis was performed with a 1-way ANOVA with a Tukey’s post hoc test. SNP, sodium nitroprusside.
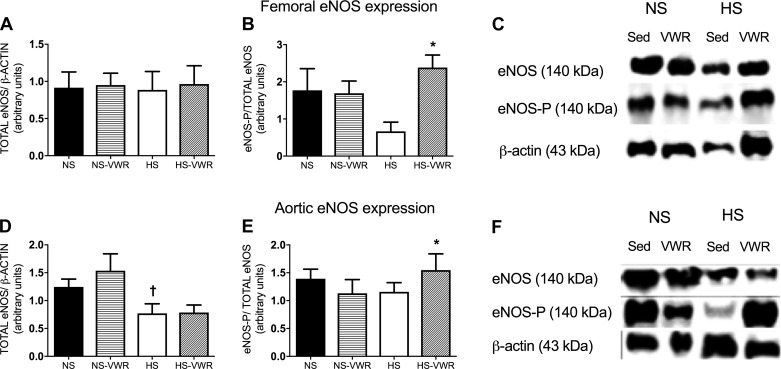
Effect of salt and wheel running on eNOS expression.
The HS-VWR group had a greater expression of femoral phosphorylated eNOS that was significant compared with the HS group; however, there was no difference in total femoral eNOS (Fig. 3, A and B). Total aortic eNOS expression was significantly elevated in the NS-VWR group in comparison to HS-fed rats; however, there was no difference between HS and HS-VWR (Fig. 3D). Consistent with femoral eNOS expression, the HS-VWR group showed a greater expression of aortic phosphorylated eNOS compared with HS alone (Fig. 3E).
Fig. 3.
A–C: total endothelial nitric oxide synthase (eNOS) protein expression in the femoral artery normalized to β-actin (A), phosphorylated eNOS (eNOS-P, ser1177) relative to total eNOS (B), and representative Western blots (C). D–F: total eNOS protein expression in the aorta normalized to β-actin (D), eNOS-P (ser1177) relative to total eNOS (E), and representative Western blots (F). Values are means ± SE; n = 9 or 10 per group. Data analysis was performed with a 1-way ANOVA with a Tukey’s post hoc test. HS, high salt; NS, normal salt; Sed, sedentary; VWR, voluntary wheel running. *vs. HS, †vs. NS-VWR (P < 0.05).
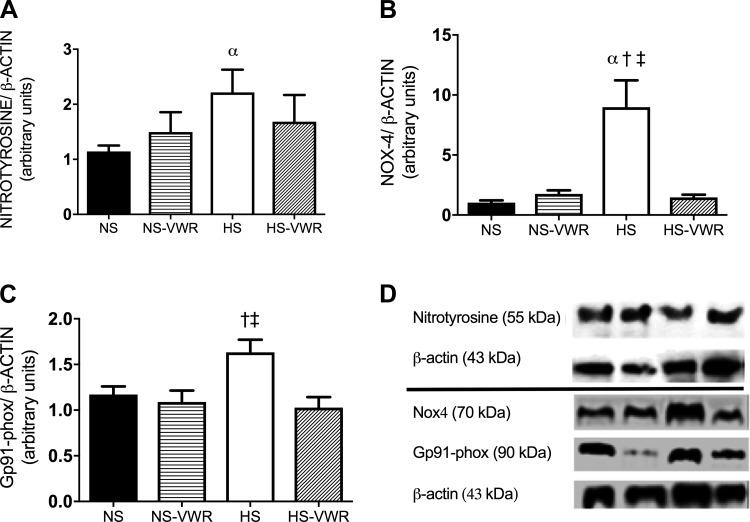
Markers of oxidative stress.
Femoral nitrotyrosine, a marker of oxidative stress, was significantly greater in the HS group compared with the NS group (Fig. 4A). Furthermore, a significant inverse relation was found between AUC for the ACh curves and nitrotyrosine (R2 = 0.47; P = 0.003). Aortic tissue was used as a surrogate for femoral tissue to measure expression of NOX4 and Gp91phox, subunits of NADPH oxidase. Aortic NOX4 expression was significantly elevated in the HS group compared with all other groups and correlated with a loss in EDR (R2 = 0.54; P = 0.010). Wheel running abolished the increase in aortic NOX4 expression in the HS group (Fig. 4B). Wheel running also attenuated the expression of aortic Gp91phox under HS conditions (Fig. 4C).
Fig. 4.
Femoral nitrotyrosine expression normalized to β-actin (A), total aortic NOX4 protein expression normalized to β-actin (B), aortic Gp91phox normalized to β-actin (C), and representative Western blots (D). Values are means ± SE; n = 8 or 9 per group. Data analysis was performed with a 1-way ANOVA with a Tukey’s post hoc test. HS, high salt; NS, normal salt; VWR, voluntary wheel running. α vs. NS, †vs. NS-VWR, ‡ vs. HS-VWR (P < 0.05).
Markers of endogenous antioxidants.
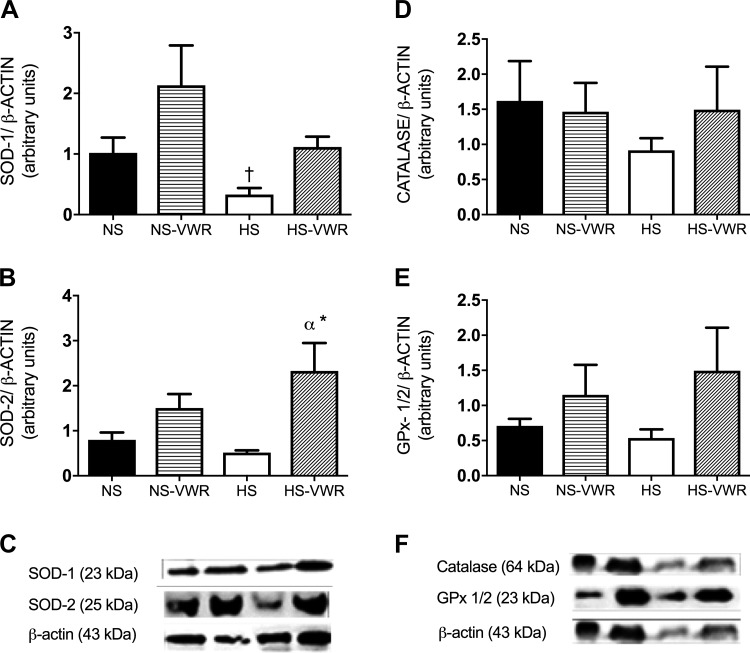
The femoral expression of SOD-1 was significantly lower on the HS diet (Fig. 5A) compared with NS-VWR; however, wheel running was not able to attenuate the effects of the HS diet. There were no other group differences for SOD-1. The expression of aortic SOD-2 is shown in Fig. 5B. SOD-2 expression was significantly greater in the HS-VWR group compared with both the NS and HS groups.
Fig. 5.
Superoxide dismutase-1 (SOD-1) protein expression in the femoral artery relative to β-actin (A), total SOD-2 protein expression in the aorta normalized to β-actin (B), representative Western blots of SOD-1 and SOD-2 (C), catalase protein expression in the aorta normalized to β-actin (D), GPx-1/2 expression in the aorta normalized to β-actin (E), and representative Western blots of catalase and GPx-1/2 (F). Values are means ± SE; n = 7 or 8 per group. Data analysis was performed with a 1-way ANOVA with a Tukey’s post hoc test. HS, high salt; NS, normal salt; VWR, voluntary wheel running. *vs. HS, α vs. NS (P < 0.05).
The aortic expressions of catalase and GPx-1/2 are shown in Fig. 5, D and E. There was no significant difference between groups for catalase or GPx-1/2 expression.
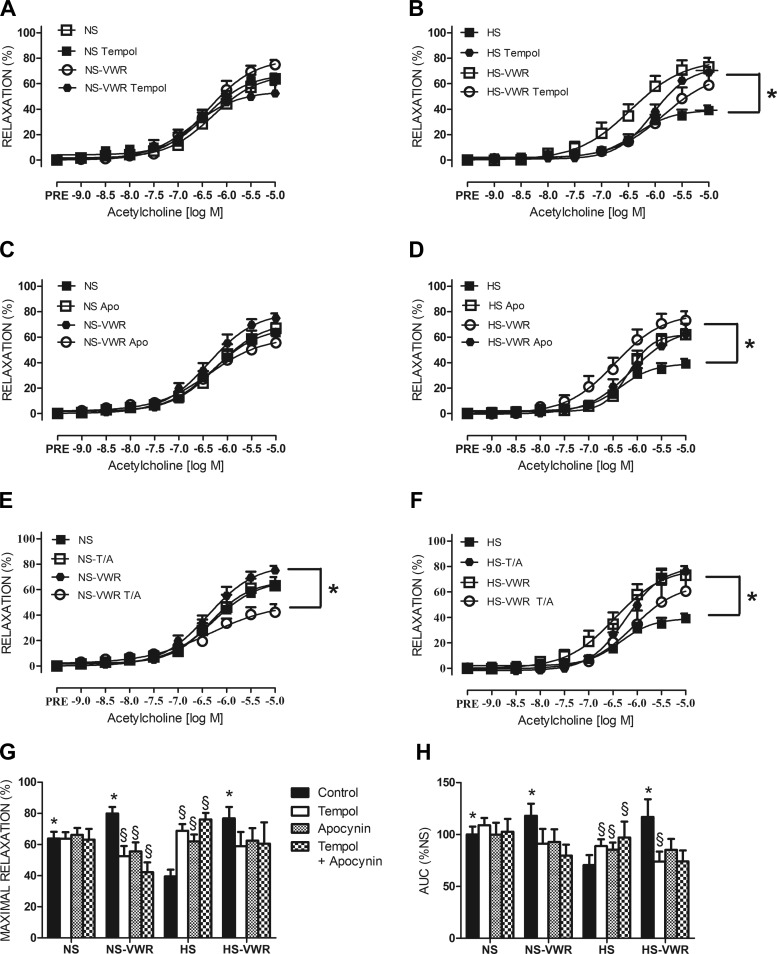
EDR response to TEMPOL.
The response of the femoral rings to increasing concentrations of ACh with and without the addition of TEMPOL in NS and HS animals is shown in Fig. 6, A and B. TEMPOL had no significant effect on relaxation (Fig. 6A) or AUC (Fig. 6H) or the logEC50 in NS rats (Table 2). However, the NS-VWR rats had a significantly lower Emax in the presence of TEMPOL (Fig. 6G).
Fig. 6.
A: endothelium-dependent relaxation (EDR) in the femoral artery in normal-salt (NS) rats in response to acetylcholine (ACh) with and without addition of the superoxide dismutase mimetic TEMPOL. B: EDR in the femoral artery in high-salt (HS) rats in response to ACh with and without the addition of TEMPOL. C: EDR in the femoral artery in NS rats in response to ACh with and without addition of the NADPH oxidase inhibitor apocynin (APO). D: EDR in femoral artery in HS rats in response to ACh with and without the addition of the NADPH oxidase inhibitor APO. E: EDR in the femoral artery in NS rats in response to ACh with and without addition of TEMPOL + APO (T/A). F: EDR in femoral artery in HS rats in response to ACh with and without the addition of T/A. G: maximal relaxation with and without the addition of TEMPOL, APO, or T/A. H: area under the curve (AUC) with and without the addition of TEMPOL, APO, or T/A. Values are means ± SE; n: NS = 12, NS-voluntary wheel running (VWR) = 13, HS = 14, HS-VWR = 12. Data analysis was performed with a (4 × 3) 2-way repeated-measures ANOVA.*vs. HS, §vs. respective control group (P < 0.05).
Table 2.
LogEC50 values
| Control | TEMPOL | Apocynin | TEMPOL + Apocynin | |
|---|---|---|---|---|
| NS | −6.2 ± 0.13 | −6.5 ± 0.15 | −6.3 ± 0.12 | −6.2 ± 0.13 |
| NS-VWR | −6.3 ± 0.18 | −6.7 ± 0.18 | −6.2 ± 0.22 | −6.8 ± 0.20 |
| HS | −6.4 ± 0.11 | −6.2 ± 0.11 | −6.1 ± 0.17 | −6.2 ± 0.18 |
| HS-VWR | −6.6 ± 0.17 | −5.3 ± 0.43* | −6.8 ± 0.38 | −6.1 ± 0.43 |
Values are means ± SE. HS, high salt; NS, normal salt; VWR, voluntary wheel running.
vs. respective control (P < 0.05).
In the HS group, treatment with TEMPOL improved relaxation (Fig. 6B), Emax (Fig. 6G), and AUC (Fig. 6H). In contrast, treatment with TEMPOL had no effect on Emax but did decrease both AUC (Fig. 6H) and logEC50 (Table 2) in the HS-VWR group.
EDR response to apocynin.
The response of the femoral rings to ACh with and without the addition of APO is shown in Fig. 6, C and D. As observed with TEMPOL administration, APO did not change relaxation in the NS group (Fig. 6C). APO administration reduced Emax in the NS-VWR group (Fig. 6G), whereas there was no change in logEC50 (Table 2) or AUC (Fig. 5H) with the addition of APO.
APO improved relaxation (Fig. 6D), Emax (Fig. 6G), and AUC (Fig. 6H) in the HS-fed animals. A trend for a decrease in logEC50 was seen in response to APO (P = 0.054; Table 2) in the HS group. APO had no significant effect on Emax, AUC, or logEC50 in the HS-VWR group (Fig. 6, G and H, Table 2).
EDR response to TEMPOL + apocynin.
The combination of TEMPOL + APO had no effect on Emax, AUC, or logEC50 in the NS group (Fig. 6, G and H, Table 2). However, the TEMPOL + APO combination significantly diminished relaxation (Fig. 6E), Emax (Fig. 6G), and AUC (Fig. 6H) in the NS-VWR group but had no effect on logEC50 (Table 2). TEMPOL + APO improved relaxation (Fig. 6F), AUC (Fig. 6H), and Emax (Fig. 6G) in the HS group, whereas no change in logEC50 (Table 2) was observed. The combination of TEMPOL + APO had no effect on any of these parameters in the HS-VWR group (Fig. 6, F–H).
DISCUSSION
The main finding of this study is that 6 wk of VWR prevented a reduction in EDR as seen in the HS-fed sedentary rats, such that the HS-VWR group was not different from the NS or NS-VWR groups. Furthermore, these findings were independent of BP, as BP did not change over the 6 wk. Regular physical activity appears to offset a salt-mediated rise in ROS production through an increase in endogenous levels of SOD-2. In addition, both femoral and aortic phosphorylated eNOS levels were greater in HS-VWR rats, likely contributing to greater NO-dependent dilation. The acute in vitro addition of the exogenous antioxidants TEMPOL and APO improved relaxation in femoral arteries from HS rats but not HS-VWR rats. In contrast, acute application of antioxidants was not beneficial and prevented wheel running-induced improvements in EDR in rats consuming a NS diet, suggesting that a balance between antioxidants and free radicals is necessary. Collectively, these findings suggest that upregulation of SOD-2 by wheel running can attenuate HS-mediated oxidative stress. Wheel running is therefore protective against the deleterious effects of HS on endothelial function in the femoral artery.
Previous studies have reported that dietary salt loading as short as 3 days can cause endothelial dysfunction without elevating BP in multiple tissue beds (striated muscle tissue, pial arterioles, and aortic tissue) (3, 17, 22, 39). Our data confirm that 6 wk of a HS diet results in endothelial dysfunction even in the absence of an elevated BP. We further extend these findings by demonstrating that 6 wk of wheel running can attenuate reductions in EDR under HS conditions in the femoral artery. The repeated shear stress that is associated with chronic exercise is thought to increase NO bioavailability, thereby improving endothelial function (6). Although losses in EDR were prevented with VWR, we found no change in relaxation in response to cumulative doses of SNP, indicating that the HS-mediated reduction in endothelial function is likely specific to the endothelium, which is consistent with other studies (17, 39, 40).
The reduction in endothelial function as a result of chronic HS consumption is thought to be due, in part, to oxidative stress (11, 16, 39, 40). Superoxide has been shown to rapidly react with NO and reduce its bioavailability, which is a well-recognized mechanism (13, 34). Several studies have demonstrated a rise in NOS uncoupling under salt loading conditions (17, 39, 40), which is a potential explanation for the impairment in endothelial function that is associated with a HS diet. Our femoral ring data demonstrate that incubation with TEMPOL or APO improves relaxation in the HS group, likely by acutely decreasing O2·− concentrations. NADPH oxidases are considered a major source of ROS including O2·− (22). However, neither TEMPOL nor APO had an effect on the NS group. This is consistent with previous work (17, 40), indicating that increased O2·− production due to HS is likely playing a role in mediating the reduction in EDR.
Prior studies have shown that HS can lower NO bioavailability in multiple tissue beds including the aorta (27, 28, 40). Therefore, we also examined the effect of HS on total and phosphorylated femoral and aortic eNOS expression and found that 6 wk of HS did not alter total femoral eNOS expression but did lower total aortic eNOS in the HS group. Although total eNOS did not change, our HS-VWR group had an elevated phosphorylated eNOS expression in both the femoral artery and aorta compared with the HS group. To our knowledge, we are the first to measure eNOS expression in the femoral artery after a chronic HS diet, whereas previous work examining total eNOS expression has predominantly used the aorta (27, 28). Similarly, a study in the Dahl salt-sensitive rat showed a salt-induced decline in aortic eNOS expression. A reduction in eNOS activity would decrease the amount of bioavailable NO, therefore contributing to the impairment of EDR present in our HS group (5, 20).
To support our functional finding that oxidative stress played a role in the salt-induced reduction in EDR, we examined femoral nitrotyrosine, a posttranslational marker of peroxynitrate (31). Nitrotyrosine expression was inversely correlated with EDR, suggesting that peroxynitrate may play a role in salt-mediated impairments in EDR. A study by Durrant et al. (9) provides support for the potential link between nitrotyrosine and salt. Young and old mice underwent chronic VWR, and a significant reduction in nitrotyrosine was only observed in the old running group. This may indicate that mice with higher levels of nitrotyrosine are more sensitive to exercise mediated changes. Previous work in the spinotrapezius arterioles and mesenteric arteries (17, 39) suggests that chronic consumption of a HS diet may increase O2·− levels through a NADPH oxidase-mediated pathway. We examined protein expression of NOX4 and Gp91phox, two subunits of NADPH oxidase. We found increased aortic NOX4 and Gp91phox expression in response to chronic HS consumption, which is consistent with previous work demonstrating a role for NADPH oxidases in HS-mediated oxidative stress (39). Furthermore, wheel running was able to protect against increased oxidative stress under HS conditions. Durrant et al. (9) demonstrated a reduction in p67phox, another subunit of NADPH oxidase, in old exercise-trained animals. Therefore, physical activity may be a potent stimulus to reduce NADPH oxidase.
Regular aerobic exercise has been shown to increase endogenous antioxidants in multiple training studies (7, 9, 35). Therefore, we sought to determine whether an increase in antioxidant protection is responsible for the decrease in ROS levels present in our wheel running HS-fed rats. Similar to previous work (35), we found that wheel running increased aortic SOD-2 in our HS group whereas femoral SOD-1 increased in the NS group in response to physical activity. SOD-2 may be the key mediating factor for our observed reductions in aortic NOX4 and Gp91phox expression under HS conditions. This is consistent with other work in which elevations in SOD-2 but not SOD-1 were associated with reductions in the Nox subunit p67phox after VWR (9). We also examined catalase and GPX1/2, two other antioxidants shown to be responsive to physical activity; however, both antioxidants were not different between groups.
When the femoral rings from the HS-VWR animals were treated with exogenous antioxidants (TEMPOL, APO, TEMPOL + APO), there was no effect on EDR. However, treatment with TEMPOL and APO independently or in combination resulted in significant reductions in EDR in our NS-VWR rats compared with NS alone. The addition of an exogenous antioxidant in vitro may have lowered the level of ROS below what is needed for cellular homeostasis and normal cellular signaling, thus acutely impairing endothelial function in NS-VWR rats. Previous work in both soleus muscle arterioles (38) and coronary arterioles (14) has shown that the acute addition of TEMPOL resulted in a decreased endothelium-dependent vasodilation in young exercise-trained rats. It has been reported that local ROS production is necessary for vasodilation (26). Kang et al. (14) speculated that exogenous TEMPOL increases the production of hydrogen peroxide (H2O2), thus overwhelming catalase, resulting in shunting of H2O2 to the hydroxyl radical and an increase in vasoconstriction. This suggests that a balance between NO and ROS is needed for proper endothelium-dependent vasodilation and warrants further investigation.
There are several limitations with the present study. The inclusion of both male and female rats would have allowed us to assess sex differences in salt-mediated changes in EDR. The femoral artery was chosen for our functional studies as it was exposed to increased shear stress as a result of VWR; however, the femoral artery is a conductance vessel and may not best represent what is happening in resistance vessels. Furthermore, because of the small amount of femoral tissue available for analysis after our functional measurements of EDR, several of our Western blot measurements were made in a surrogate tissue, the aorta. This may not represent what is occurring in the femoral artery; however, other studies have shown similar decrements in endothelial function in the aorta (40), the spinotrapezuis arterioles (16), and the pial artery (22) in response to a HS diet. Finally, to compare the salt content of rodent to human diet studies, it is important to note that most human intervention studies increase the sodium consumption by 40–50 times the minimal requirement for salt (4), whereas in rat studies the salt content can range from 60 to 170 times more than estimated requirements. As we used a 4% NaCl diet, we fall toward the lower end of that range. Furthermore, there are species differences in metabolic rate and sodium turnover in the body that can make a direct comparison challenging (4).
In conclusion, 6 wk of physical activity protected HS-fed rats from reductions in endothelial function, likely through increased SOD-2 and reduced oxidative stress. Importantly, this effect was independent of BP. The decline in EDR under HS conditions appears to be linked to an increase in nitrotyrosine, as it correlated with a reduction in AUC and both Nox4 and Gp91phox expression were significantly elevated in the presence of HS. Wheel running increased SOD-2, and acute in vitro incubation with the antioxidants TEMPOL and APO improved EDR in a fashion similar to wheel running but no additive benefit was observed. Finally, the addition of exogenous antioxidants appears to reduce endothelial function in wheel running rats fed a NS diet. This may suggest a critical level of ROS needed for proper endothelial function.
GRANTS
This work was supported by an American College of Sports Medicine Doctoral Student Research Grant (J. J. Guers) and National Institute of General Medical Sciences Grant P20 GM-113125 (S. L. Lennon).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.L.L., W.B.F., and D.G.E. conceived and designed research; J.J.G. and L.K.-L. performed experiments; J.J.G. and S.L.L. analyzed data; J.J.G., L.K.-L., W.B.F., D.G.E., and S.L.L. interpreted results of experiments; J.J.G. and S.L.L. prepared figures; J.J.G. drafted manuscript; J.J.G., L.K.-L., W.B.F., D.G.E., and S.L.L. edited and revised manuscript; J.J.G., L.K.-L., W.B.F., D.G.E., and S.L.L. approved final version of manuscript.
ACKNOWLEDGMENTS
The experiments of this study were conducted in the Department of Kinesiology and Applied Physiology at the University of Delaware.
REFERENCES
- 1.Aburto NJ, Ziolkovska A, Hooper L, Elliott P, Cappuccio FP, Meerpohl JJ. Effect of lower sodium intake on health: systematic review and meta-analyses. BMJ 346: f1326, 2013. doi: 10.1136/bmj.f1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blair SN, Kohl HW 3rd, Paffenbarger RS Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA 262: 2395–2401, 1989. doi: 10.1001/jama.1989.03430170057028. [DOI] [PubMed] [Google Scholar]
- 3.Boegehold MA. Effect of dietary salt on arteriolar nitric oxide in striated muscle of normotensive rats. Am J Physiol Heart Circ Physiol 264: H1810–H1816, 1993. doi: 10.1152/ajpheart.1993.264.6.H1810. [DOI] [PubMed] [Google Scholar]
- 4.Boegehold MA. The effect of high salt intake on endothelial function: reduced vascular nitric oxide in the absence of hypertension. J Vasc Res 50: 458–467, 2013. doi: 10.1159/000355270. [DOI] [PubMed] [Google Scholar]
- 5.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res 87: 840–844, 2000. doi: 10.1161/01.RES.87.10.840. [DOI] [PubMed] [Google Scholar]
- 6.Campos JC, Gomes KM, Ferreira JC. Impact of exercise training on redox signaling in cardiovascular diseases. Food Chem Toxicol 62: 107–119, 2013. doi: 10.1016/j.fct.2013.08.035. [DOI] [PubMed] [Google Scholar]
- 7.de Moraes C, Davel AP, Rossoni LV, Antunes E, Zanesco A. Exercise training improves relaxation response and SOD-1 expression in aortic and mesenteric rings from high caloric diet-fed rats. BMC Physiol 8: 12, 2008. doi: 10.1186/1472-6793-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DuPont JJ, Greaney JL, Wenner MM, Lennon-Edwards SL, Sanders PW, Farquhar WB, Edwards DG. High dietary sodium intake impairs endothelium-dependent dilation in healthy salt-resistant humans. J Hypertens 31: 530–536, 2013. doi: 10.1097/HJH.0b013e32835c6ca8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durrant JR, Seals DR, Connell ML, Russell MJ, Lawson BR, Folian BJ, Donato AJ, Lesniewski LA. Voluntary wheel running restores endothelial function in conduit arteries of old mice: direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. J Physiol 587: 3271–3285, 2009. doi: 10.1113/jphysiol.2009.169771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288: 373–376, 1980. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 11.Greaney JL, DuPont JJ, Lennon-Edwards SL, Sanders PW, Edwards DG, Farquhar WB. Dietary sodium loading impairs microvascular function independent of blood pressure in humans: role of oxidative stress. J Physiol 590: 5519–5528, 2012. doi: 10.1113/jphysiol.2012.236992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green DJ, Maiorana A, O’Driscoll G, Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol 561: 1–25, 2004. doi: 10.1113/jphysiol.2004.068197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gryglewski RJ, Palmer RM, Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature 320: 454–456, 1986. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- 14.Kang LS, Reyes RA, Muller-Delp JM. Aging impairs flow-induced dilation in coronary arterioles: role of NO and H2O2. Am J Physiol Heart Circ Physiol 297: H1087–H1095, 2009. doi: 10.1152/ajpheart.00356.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci USA 88: 4651–4655, 1991. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lenda DM, Boegehold MA. Effect of a high-salt diet on oxidant enzyme activity in skeletal muscle microcirculation. Am J Physiol Heart Circ Physiol 282: H395–H402, 2002. doi: 10.1152/ajpheart.0354.2001. [DOI] [PubMed] [Google Scholar]
- 17.Lenda DM, Sauls BA, Boegehold MA. Reactive oxygen species may contribute to reduced endothelium-dependent dilation in rats fed high salt. Am J Physiol Heart Circ Physiol 279: H7–H14, 2000. doi: 10.1152/ajpheart.2000.279.1.H7. [DOI] [PubMed] [Google Scholar]
- 18.Lennon-Edwards S, Ramick MG, Matthews EL, Brian MS, Farquhar WB, Edwards DG. Salt loading has a more deleterious effect on flow-mediated dilation in salt-resistant men than women. Nutr Metab Cardiovasc Dis 24: 990–995, 2014. doi: 10.1016/j.numecd.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lesniewski LA, Zigler ML, Durrant JR, Nowlan MJ, Folian BJ, Donato AJ, Seals DR. Aging compounds western diet-associated large artery endothelial dysfunction in mice: prevention by voluntary aerobic exercise. Exp Gerontol 48: 1218–1225, 2013. doi: 10.1016/j.exger.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li H, Horke S, Förstermann U. Vascular oxidative stress, nitric oxide and atherosclerosis. Atherosclerosis 237: 208–219, 2014. doi: 10.1016/j.atherosclerosis.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation 105: 1135–1143, 2002. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Rusch NJ, Lombard JH. Loss of endothelium and receptor-mediated dilation in pial arterioles of rats fed a short-term high salt diet. Hypertension 33: 686–688, 1999. doi: 10.1161/01.HYP.33.2.686. [DOI] [PubMed] [Google Scholar]
- 23.Loperena R, Harrison DG. Oxidative stress and hypertensive diseases. Med Clin North Am 101: 169–193, 2017. doi: 10.1016/j.mcna.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lüscher TF, Tanner FC, Tschudi MR, Noll G. Endothelial dysfunction in coronary artery disease. Annu Rev Med 44: 395–418, 1993. doi: 10.1146/annurev.me.44.020193.002143. [DOI] [PubMed] [Google Scholar]
- 25.Martens CR, Kuczmarski JM, Kim J, Guers JJ, Harris MB, Lennon-Edwards S, Edwards DG. Voluntary wheel running augments aortic l-arginine transport and endothelial function in rats with chronic kidney disease. Am J Physiol Renal Physiol 307: F418–F426, 2014. doi: 10.1152/ajprenal.00014.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marvar PJ, Nurkiewicz TR, Boegehold MA. Reduced arteriolar responses to skeletal muscle contraction after ingestion of a high salt diet. J Vasc Res 42: 226–236, 2005. doi: 10.1159/000085461. [DOI] [PubMed] [Google Scholar]
- 27.Ni Z, Oveisi F, Vaziri ND. Nitric oxide synthase isotype expression in salt-sensitive and salt-resistant Dahl rats. Hypertension 34: 552–557, 1999. doi: 10.1161/01.HYP.34.4.552. [DOI] [PubMed] [Google Scholar]
- 28.Ni Z, Vaziri ND. Effect of salt loading on nitric oxide synthase expression in normotensive rats. Am J Hypertens 14: 155–163, 2001. doi: 10.1016/S0895-7061(00)01234-6. [DOI] [PubMed] [Google Scholar]
- 29.Nurkiewicz TR, Boegehold MA. High salt intake reduces endothelium-dependent dilation of mouse arterioles via superoxide anion generated from nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol 292: R1550–R1556, 2007. doi: 10.1152/ajpregu.00703.2006. [DOI] [PubMed] [Google Scholar]
- 30.Poggio R, Gutierrez L, Matta MG, Elorriaga N, Irazola V, Rubinstein A. Daily sodium consumption and CVD mortality in the general population: systematic review and meta-analysis of prospective studies. Public Health Nutr 18: 695–704, 2015. doi: 10.1017/S1368980014000949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Radi R. Nitric oxide, oxidants, and protein tyrosine nitration. Proc Natl Acad Sci USA 101: 4003–4008, 2004. doi: 10.1073/pnas.0307446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roque FR, Briones AM, García-Redondo AB, Galán M, Martínez-Revelles S, Avendaño MS, Cachofeiro V, Fernandes T, Vassallo DV, Oliveira EM, Salaices M. Aerobic exercise reduces oxidative stress and improves vascular changes of small mesenteric and coronary arteries in hypertension. Br J Pharmacol 168: 686–703, 2013. doi: 10.1111/j.1476-5381.2012.02224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rubanyi GM. Endothelium-derived relaxing and contracting factors. J Cell Biochem 46: 27–36, 1991. doi: 10.1002/jcb.240460106. [DOI] [PubMed] [Google Scholar]
- 34.Rubanyi GM, Vanhoutte PM. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am J Physiol Heart Circ Physiol 250: H822–H827, 1986. doi: 10.1152/ajpheart.1986.250.5.H822. [DOI] [PubMed] [Google Scholar]
- 35.Rush JW, Turk JR, Laughlin MH. Exercise training regulates SOD-1 and oxidative stress in porcine aortic endothelium. Am J Physiol Heart Circ Physiol 284: H1378–H1387, 2003. doi: 10.1152/ajpheart.00190.2002. [DOI] [PubMed] [Google Scholar]
- 36.Savage MV, Mackie GF, Bolter CP. Effect of exercise on the development of salt-induced hypertension in Dahl-S rats. J Hypertens 4: 289–293, 1986. doi: 10.1097/00004872-198606000-00006. [DOI] [PubMed] [Google Scholar]
- 37.Shepherd RE, Kuehne ML, Kenno KA, Durstine JL, Balon TW, Rapp JP. Attenuation of blood pressure increases in Dahl salt-sensitive rats by exercise. J Appl Physiol 52: 1608–1613, 1982. doi: 10.1152/jappl.1982.52.6.1608. [DOI] [PubMed] [Google Scholar]
- 38.Sindler AL, Reyes R, Chen B, Ghosh P, Gurovich AN, Kang LS, Cardounel AJ, Delp MD, Muller-Delp JM. Age and exercise training alter signaling through reactive oxygen species in the endothelium of skeletal muscle arterioles. J Appl Physiol (1985) 114: 681–693, 2013. doi: 10.1152/japplphysiol.00341.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu J, Huang T, Lombard JH. Effect of high-salt diet on vascular relaxation and oxidative stress in mesenteric resistance arteries. J Vasc Res 44: 382–390, 2007. doi: 10.1159/000102955. [DOI] [PubMed] [Google Scholar]
- 40.Zhu J, Mori T, Huang T, Lombard JH. Effect of high-salt diet on NO release and superoxide production in rat aorta. Am J Physiol Heart Circ Physiol 286: H575–H583, 2004. doi: 10.1152/ajpheart.00331.2003. [DOI] [PubMed] [Google Scholar]