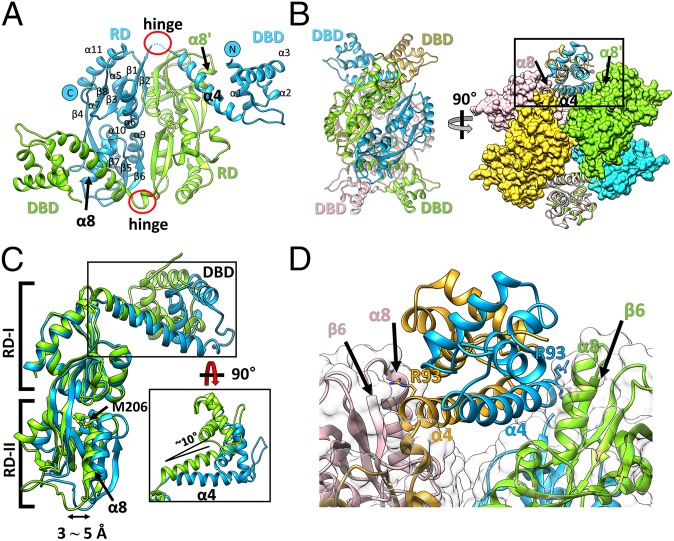
Fig. 2.
Overall structure of wild-type full-length StHypT. (A) The structure of the asymmetric unit containing two protomers (chain A in green, chain B in cyan). The N and C termini and the secondary structural elements are labeled on chain B. (B) Two orthogonal views of the tetramer structure by combining two asymmetric units related to the crystallographic twofold symmetry. (Left) The four promoters are shown in ribbon representation with different colors (green, cyan, yellow, and pink). (Right) RDs are shown in the surface representations, and DBDs are in the ribbon diagram. The contact region between α4 in the DBD and α8 in RD-II is marked as a box, which is enlarged in D. The yellow and pink protomers are symmetry-related molecules to the green and cyan protomers, respectively. (C) Structural superposition of two protomers using the RDs as references. The DBDs have 10° angular variation (black box), and RD-II has a 3∼5 Å translational shift between two protomers at α8. (D) A close-up view of the box region of B. Each linker helix (α4) makes direct contact with α8 in RD-II of the other protomer in the asymmetric unit. Concomitantly, the Arg93 residues in α4 (stick representation) interact with the loop between α8 and β6 in RD-II.