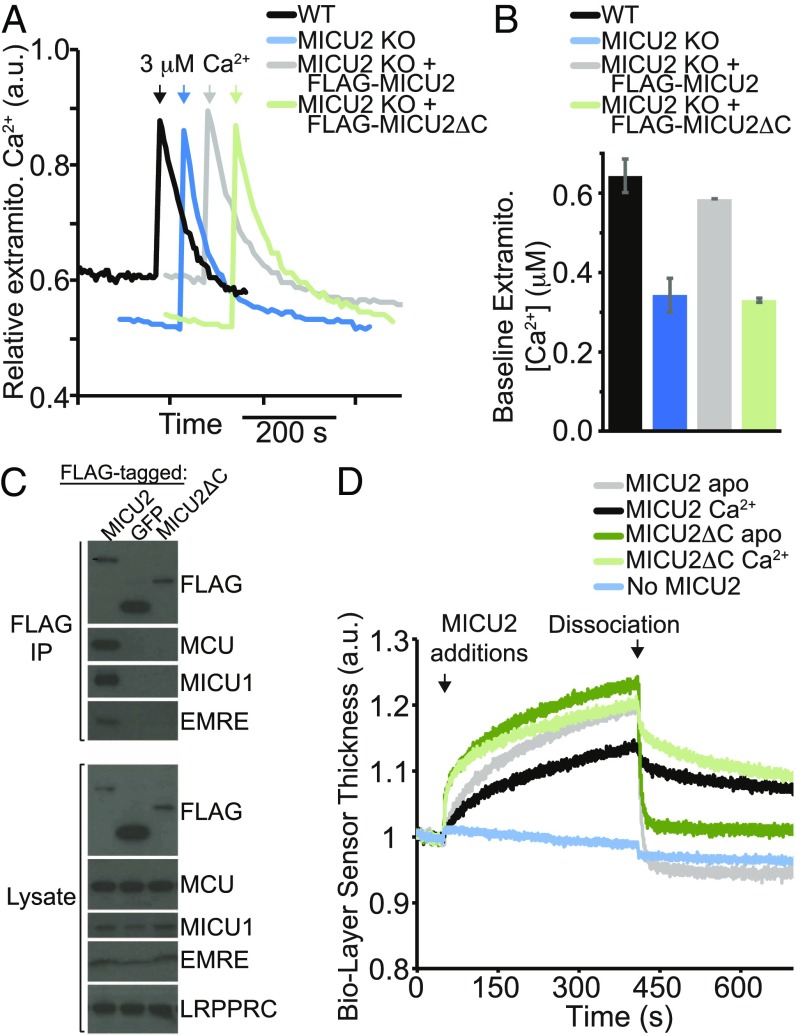
Fig. 4.
MICU2 C-terminal helix is required for function and interaction with the uniporter in HEK-293T cells in situ, but not for MICU1–MICU2 interaction in vitro. (A) Extramitochondrial calcium levels in digitonin-permeabilized HEK-293T cells (WT), HEK-293T cells with MICU2 knocked out (MICU2 KO), and MICU2 KO cells with FLAG-MICU2 or FLAG-MICU2 with the C-terminal α-helix deleted (FLAG-MICU2ΔC). At the indicated time, a 3 µM pulse of CaCl2 was given. Fluorescence signal is normalized on a scale of 0–1. (B) The extramitochondrial Ca2+ concentration before the 3 µM pulse of CaCl2 (“baseline”) is quantified 10 min after cell permeabilization across biological replicates and mean ± SEM is reported (n = 3). (C) FLAG coimmunoprecipitation to probe the interaction between FLAG-tagged MICU2, MICU2ΔC, or a negative control mito-targeted GFP and other components of the uniporter complex (MCU, MICU1, and EMRE) in MICU2 KO cells. A representative experiment is shown. (D) Biolayer interferometry traces showing MICU2 or MICU2ΔC binding to the streptavidin-coated ForteBio tips loaded with biotinylated MICU1ΔC in a buffer containing Ca2+ or EGTA. Dissociation begins at the indicated time point, when the well solution for the tips is changed to buffer lacking the MICU2 protein. Signal is corrected for nonspecific binding by subtracting signal obtained from tips lacking the addition of biotinylated MICU1.