Significance
Despite great advances in therapy for breast cancer, more than half of patients still face tumor recurrence and drug resistance. Therefore, a better understanding of tumor progression and drug resistance is needed for developing next-generation therapies. Here, we report the role of galactosyltransferase β3GalT5, a key enzyme responsible for the biosynthesis of SSEA3 which is then converted to SSEA4 and Globo-H. We demonstrated that knockdown of β3GalT5 would destruct the complex formation of SSEA3/SSEA4/Globo-H with FAK/CAV1/AKT/RIP and cause the dissociation of RIP from the complex to interact with FADD, thus triggering cancer cell apoptosis and suppressing metastasis. These findings provide a strategy of therapeutics for breast cancer as demonstrated by the combination use of antibodies against Globo-H and SSEA4.
Keywords: β3GalT5, SSEA3, SSEA4, Globo-H, FAK
Abstract
The globo-series glycosphingolipids (GSLs) SSEA3, SSEA4, and Globo-H specifically expressed on cancer cells are found to correlate with tumor progression and metastasis, but the functional roles of these GSLs and the key enzyme β1,3-galactosyltransferase V (β3GalT5) that converts Gb4 to SSEA3 remain largely unclear. Here we show that the expression of β3GalT5 significantly correlates with tumor progression and poor survival in patients, and the globo-series GSLs in breast cancer cells form a complex in membrane lipid raft with caveolin-1 (CAV1) and focal adhesion kinase (FAK) which then interact with AKT and receptor-interacting protein kinase (RIP), respectively. Knockdown of β3GalT5 disrupts the complex and induces apoptosis through dissociation of RIP from the complex to interact with the Fas death domain (FADD) and trigger the Fas-dependent pathway. This finding provides a link between SSEA3/SSEA4/Globo-H and the FAK/CAV1/AKT/RIP complex in tumor progression and apoptosis and suggests a direction for the treatment of breast cancer, as demonstrated by the combined use of antibodies against Globo-H and SSEA4.
The aberrant glycosylation and elevated expression of specific glycosphingolipids (GSLs) on the cell surface of different types of cancers has led to the development of new anticancer agents (1, 2). We have previously reported that the globo-series GSLs, including SSEA3, SSEA4, and Globo-H, are highly and specifically expressed on the cell surface of 15 types of cancers, and SSEA3 is a cancer-specific marker useful for the detection and enrichment of breast cancer stem cells, and only 10 SSEA3+-CD44+-CD24− cells are sufficient to support tumor formation (3, 4).
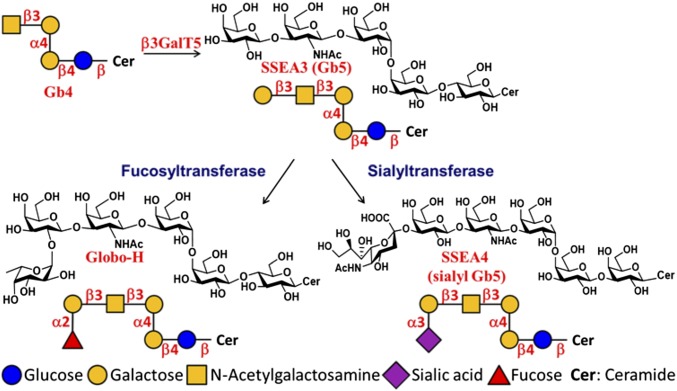
These globo-series GSLs are synthesized from globoside-4 (Gb4) by the key enzyme β1,3-galactosyltransferase V (β3GalT5), which catalyzes the galactosylation of Gb4 to form Gb5 (also called SSEA3) (5). SSEA3 is then converted to SSEA4 catalyzed by a β-galactoside α2,3-sialyltransferase, ST3Gal-II (6) and to Globo-H by an α1,2-fucosyltransferase-1, FUT-1 (7) or FUT-2 (8); so, the expression of β3GalT5 will determine the levels of these three cancer-related globo-series GSLs (Scheme 1).
Scheme 1.
The biosynthetic pathway of SSEA3, Globo-H, and SSEA4, showing the role of β3GalT5 in the pathway.
SSEA3 has a higher percentage of expression (77.5%) than Globo-H (61%) in clinical breast cancer specimens (2) and in nonsmall cell lung cancer cells with multiple drug resistance (9), and the potential of tumorigenesis and sphere formation of colorectal cancer cells is enhanced with increasing SSEA3 expression (10). In addition, the enzymatic activity of β3GalT5 in the sera from clinical ovarian cancer and uterine cervical cancer patients is associated with cancer progression (11), and the expression of β3GalT5 is up-regulated in metastatic hepatocellular carcinoma HCCLM3 cells (12) and liver cancer-initiating cells (13). We also find that knockdown of β3GalT5 inhibited cell proliferation and induced caspase-3–mediated apoptosis in breast cancer cells (4), suggesting that the tumorigenicity and multidrug resistance of cancer cells may correlate with the expression of SSEA3.
It has been shown that the globo-series GSLs are located in the GSL-enriched microdomain (GEM), and the invasive properties of MCF-7 are highly correlated with the clustering of SSEA4 and the subsequent activation of focal adhesion kinase (FAK) in GEM (14, 15). In addition, FAK, a tyrosine kinase, is significantly up-regulated in triple-negative or metastatic breast cancer tissues and is related to cancer recurrence (16, 17). Knockdown of FAK is found to induce apoptosis in breast cancer cells, possibly through the suppression of FAK-mediated signaling (18). Displacement of FAK by its dominant-negative form (FAK-c-terminal domain, or FAK-CD) can induce cell apoptosis, and the disruption of FAK-mediated signaling is associated with the initiation of Fas signaling (19). It is also found that when caspase-3 and -8 are activated to cleave FAK, the survival signaling mediated by FAK is diminished (20). In addition, RIP, a death domain-containing Ser/Thr kinase in the Fas signaling pathway, is involved in the apoptosis triggered by attenuation of FAK; and the association of RIP and FAK results in cell survival by inhibiting the recruitment of RIP to FADD, suggesting a regulatory cross-talk between Fas-induced apoptotic and FAK-mediated survival signaling (21, 22).
Here, we first examined the clinical relevance of β3GalT5 expression and breast cancer progression. We found that SSEA3, FAK, and CAV1 existed as a complex to maintain the survival of breast cancer cells, and after knockdown of β3GalT5, the association of RIP and FAK was disrupted, while the interaction of RIP with FADD increased. These observations led us to further investigate the signaling pathway associated with the globo-series GSLs to better understand the roles of β3GalT5 and the globo-series GSLs in cell survival and apoptosis.
Results
Clinical Relevance of β3GalT5 in Patients with Breast Cancer.
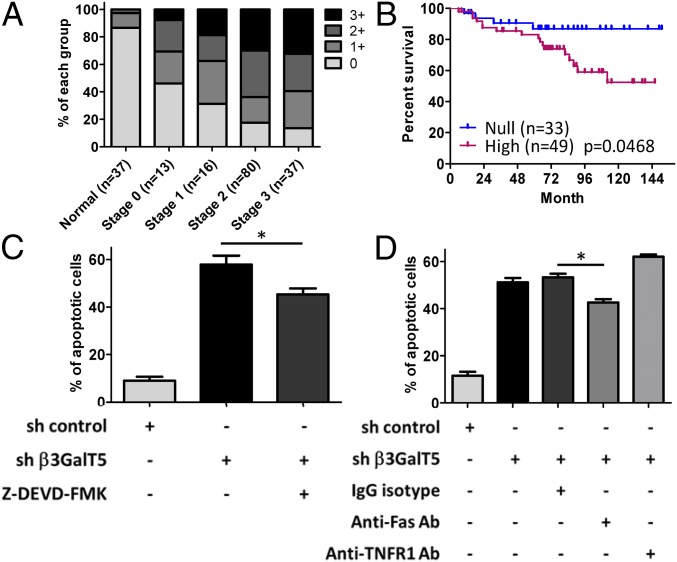
Since SSEA3, SSEA4, and Globo-H are highly and specifically expressed on the surface of cancer cells, it is likely that the enzyme β3GalT5 is key to the expression of these three cancer-related globo-series GSLs (Scheme 1). To understand the correlation of β3GalT5 expression and the stages of breast cancer, we performed immunohistochemistry (IHC) on stage 0–3 breast carcinoma specimens and normal breast tissue sections (SI Appendix, Fig. S1). The data indicated that β3GalT5 was highly expressed in the cytoplasm of breast carcinoma cells, while most normal breast tissue sections were β3GalT5-negative (Fig. 1A). Moreover, the statistical results showed that 58.3% of breast carcinoma specimens were β3GalT5-positive in stage 0, and 86.5% were intensely stained in stage 3 specimens. In contrast, 86.4% of normal breast tissue specimens were negatively stained. This result indicated that β3GalT5 was explicitly expressed in breast carcinoma tissues and positively correlated with the stages of breast carcinoma and was consistent with the observation that the globo-series GSLs were generally not expressed on normal cell lines (SI Appendix, Table S1). The disease-free survival rate in the patients with over 10 y follow-up was stratified based on the IHC scores of β3GalT5 expression: the intensity of β3GalT5 scored as 0 was categorized as the “null” group, whereas the staining score of 3 was categorized as the “high” group (Fig. 1B). As shown in the data, there was a significant difference in the disease-free survival rate between the “null” and the “high” groups, and the high expression level of β3GalT5 correlated with poor disease-free survival in breast cancer patients.
Fig. 1.
Expression of β3GalT5 is associated with breast cancer progression. (A) Immunohistochemistry of β3GalT5: normal breast tissues (n = 37), tissues of stage 0 (n = 13), stage 1 (n = 16), stage 2 (n = 80), and stage 3 (n = 37) were stained with hematoxylin after immunohistochemistry. The staining intensity of normal and cancer tissues was scored as 0 (negative), 1+ (weak), 2+ (moderate), and 3+ (strong). (B) The expression level of β3GalT5 in breast carcinoma correlates with disease-free survival. Shown is the results of 82 patients with β3GalT5 staining intensity scores of 0 (null) and 3 (high). All P values were calculated by log-rank (Mantel–Cox) test. (C) The percentage of apoptotic MDA-MB-231 cells with knockdown of β3GalT5 or overexpression of β3GalT5. (D) The apoptotic MDA-MB-231 cells with β3GalT5 knockdown were treated with anti-Fas or anti-TNFR antibody. Representative data among triplicated experiments are shown. *P < 0.05; n s., not significant.
Further analysis of β3GalT5 expression and pathological factors revealed that β3GalT5 is significantly associated with progressive clinical stages (P = 0.003) and lymph node metastasis (P = 0.0259) (SI Appendix, Table S2). There was no significant correlation between the expression of β3GalT5 and patients’ age, tumor status, distal metastasis, expression of estrogen receptor (ER), progesterone receptor (PR), or human epidermal growth factor receptor 2 (Her2), or recurrence.
Knockdown of β3GalT5 Induced Cell Apoptosis and Reduced Cell Migration and Invasion.
Since knockdown of β3GalT5 caused a significant reduction of SSEA3 expression and resulted in the activation of apoptosis through caspase-8 and -3 activations (4), we further analyzed the expressions of SSEA3, SSEA4, and Globo-H in β3GalT5 knockdown cells by flow cytometry and LC-MS/MS. The results showed that these three globo-series GSLs were nearly not detectable after knockdown of β3GalT5 in cancer cells (SI Appendix, Fig. S2 A and B). Moreover, knockdown of β3GalT5 in MDA-MB-231 cells caused apoptosis, and introduction of β3GalT5 to these cells increased cell viability (Fig. 1C) and partially restored the expression of SSEA3 and Globo-H on cell surface (SI Appendix, Fig. S3 A and B). To investigate if cell apoptosis is through the Fas or TNFRI signaling pathway, we added anti-Fas or anti-TNFRI neutralizing antibody to block the signaling of MDA-MB-231 breast cancer cells after knockdown of β3GalT5 (Fig. 1D). We found that treatment with anti-Fas antibody significantly reduced the percentage of apoptotic cells, whereas no change was observed in the treatment with anti-TNFRI antibody and isotype control, suggesting that the Fas signaling pathway is associated with the apoptosis induced by β3GalT5 knockdown.
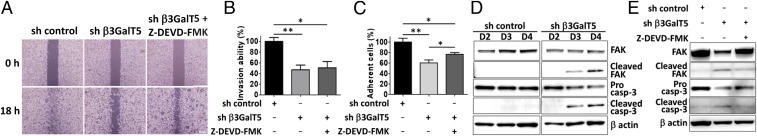
To further investigate if β3GalT5 plays a role in tumor metastasis, the assays for cellular migration, invasion, and adhesion were performed in MDA-MB-231 breast cancer cells with β3GalT5 knockdown. It was found that knockdown of β3GalT5 significantly suppressed the abilities of migration, adhesion, and invasion of MDA-MB-231 cells compared with the control cells (Fig. 2 A–C). FAK is associated with cancer cell migration and metastasis because the cleavage of FAK by activated caspase-3 will lead to the inhibition of cell migration (20). So, we measured the protein level of FAK and its cleaved fragment p33 (Fig. 2D) in the β3GalT5 knockdown cells to investigate whether the reduced metastasis capability was due to the decreased expression of FAK. The results showed that knockdown of β3GalT5 activated caspase-3 and significantly reduced the level of FAK, with a concurrent appearance of the fragment FAK p33, and treatment with a caspase-3 inhibitor restored the expression of FAK (Fig. 2E). To further confirm if the reduced cell migration and adhesion with β3GalT5 knockdown was related to caspase-3 activation, the caspase-3 inhibitor z-DEVD was added to inhibit cell apoptosis; however, the migration and adhesion of cells were still observed, suggesting that β3GalT5 probably plays a role in cell metastasis and malignancy through multiple pathways.
Fig. 2.
Knockdown of β3GalT5 caused cell apoptosis and inhibition of cell metastasis. (A) The migration ability of MDA-MB-231 cells measured by wound-healing assay. Cells infected with sh β3GalT5 were treated with or without Z-DEVD-FMK, a caspase-3 inhibitor, and incubated for 18 h. Representative data among triplicated experiments are shown. (Scale bars, 100 μm.) (B and C) The invasion (B) and adhesion (C) abilities of MDA-MB-231 cells with or without β3GalT5 knockdown. The percentage of invaded cells were measured with calcium AM. (D) Western blot analysis of caspase-3 activation and cleavage of FAK in MDA-MB-231 cells with β3GalT5 knockdown. (E) Expression of caspase-3 and FAK in MDA-MB-231 cells in the presence or absence of caspase-3 inhibitor. Bars represent the mean ± SD values from three experiments. The P value was obtained by t test. *P < 0.05; **P < 0.01.
SSEA3 Cooperated with FAK for Survival of Cancer Cells.
FAK is reported to have direct association with AKT for promoting cell adhesion and metastatic abilities (23), but the relationship between SSEA3 and FAK in cancer progression is unknown. Here, we found that the expression and phosphorylation of AKT was suppressed in MDA-MB-231 cells with β3GalT5 knockdown (SI Appendix, Fig. S4 A and B). We further examined the correlation of FAK and AKT by immunoprecipitation (SI Appendix, Fig. S4C) and found that knockdown of β3GalT5 caused the dissociation of FAK and AKT in MDA-MB-231 cells. Conversely, inhibition of caspase-3 was able to significantly restore the association between FAK and AKT. These results suggested that the globo-series GSLs were positively correlated with the expression of FAK and the AKT survival pathway. To confirm if the expression of globo-series GSLs correlated with FAK expression in cancer cells, we knocked down FAK in breast cancer cells. As shown in SI Appendix, Fig. S5A, both β3GalT5 knockdown cells and FAK knockdown cells showed a reduction of FAK expression in MDA-MB-231 cells compared with their controls, whereas knockdown of β3GalT5 had no effect on the FAK mRNA level (SI Appendix, Fig. S5 B–D). Interestingly, we observed that the SSEA3 expression was significantly reduced from 56.5 to 19.4% in FAK-silenced breast cancer cells compared with the control cells. Moreover, when the FAK-silenced cells were treated with triton X-100 for intracellular staining, the cells showed an increase in SSEA3 expression (SI Appendix, Fig. S5E), suggesting that the expression of FAK is in parallel with SSEA3 expression on the surface of breast cancer cells. Treatment with PF537228, an inhibitor of FAK phosphorylation at Tyr397, had no effect on the expression levels of SSEA3, SSEA4, or Globo-H on the cell surface (SI Appendix, Fig. S5F), indicating that the phosphorylation of FAK did not affect the expression of globo-series GSLs.
SSEA3/Globo-H/FAK and SSEA3/SSEA4/CAV1 Complexes in Breast Cancer Cells.
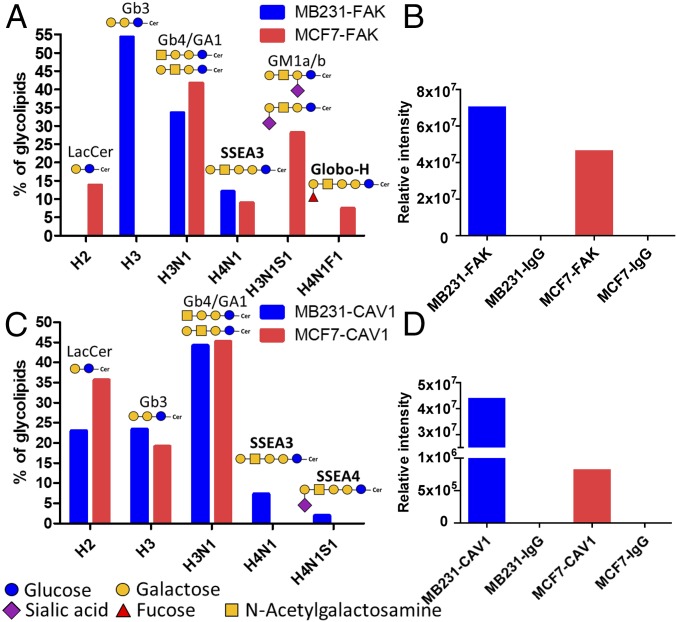
Both FAK and SSEA3 are found in GEM (15), but whether FAK and SSEA3 form a complex in GEM is not explored. To investigate whether SSEA3 associates with FAK and the signaling, we performed immunoprecipitation in MDA-MB-231 and MCF7 cell lysates with anti-SSEA3 antibody. The results showed that the MDA-MB-231 and MCF7 immunoprecipitates were strongly positive in SSEA3 and FAK, and the level of FAK protein was higher in MDA-MB-231 cells (SI Appendix, Fig. S5 G and H). Interestingly, the relative percentages of SSEA4 in MDA-MB-231 cells were higher than that in MCF7 cells, but the percentage of Globo-H in MCF7 cells was higher than that in MDA-MB-231 cells. These results suggested that SSEA3 (and to a lesser extent Globo-H) and FAK form a cluster on breast cancer cells. In addition, when the FAK protein from these two cell lysates was immunoprecipitated by anti-FAK antibody and subject to glycolipid profiling and protein identification (Fig. 3 A and B), the results showed that the anti-FAK antibody pulled down more SSEA3 from MDA-MB-231 cells than from MCF7 cells which showed more Globo-H than MDA-MB231. Consistent with the anti-SSEA3 pulldown results, MDA-MB-231 cells showed higher SSEA3 and FAK interaction than MCF7 cells, indicating that SSEA3 was explicitly more associated with FAK in MDA-MB-231 cells for tumor progression.
Fig. 3.
Identification of glycolipids in breast cancer cell lysates via coimmunoprecipitation with FAK or CAV1 antibody. (Aand C) The percentage of globo-series GSLs determined from anti-FAK (A) and anti-CAV1 (C) pulldown by LC-MS/MS in MCF7 and MDA-MB-231 cells. (F, Fucose; H, Hexose; N, N-acetyl-Hexosamine; S, Sialic acid) (B and D) Quantitation of FAK (B) and CAV1 (D) peptides from the immunoprecipitates of MCF7 and MDA-MB-231 cells by LC-MS/MS. All glycolipids are with C16:0 fatty acid in all examinations.
The membrane protein CAV1 is a lipid raft marker and can promote the stabilization of FAK within focal adhesion for tumor metastasis. To examine the possible association between CAV1 and SSEA3/SSEA4/Globo-H in MDA-MB-231 and MCF7 cells, the CAV1 protein from these two cell lysates was immunoprecipitated by anti-CAV1 antibody. Interestingly, we observed that the anti-CAV1 antibody pulled down more SSEA3 and SSEA4 glycolipids in MDA-MB-231 cells compared with MCF7 cells (Fig. 3C). The CAV1 complex was then analyzed and quantified by mass spectrometry to confirm the protein level of CAV1, which was higher in MDA-MB-231 cells than in MCF7 cells (Fig. 3D). Taken together, these results indicated that SSEA3/SSEA4 was associated with CAV1 in breast cancer cells.
Colocalization of SSEA3, CAV1, and FAK on Membrane Raft of Breast Cancer Cells.
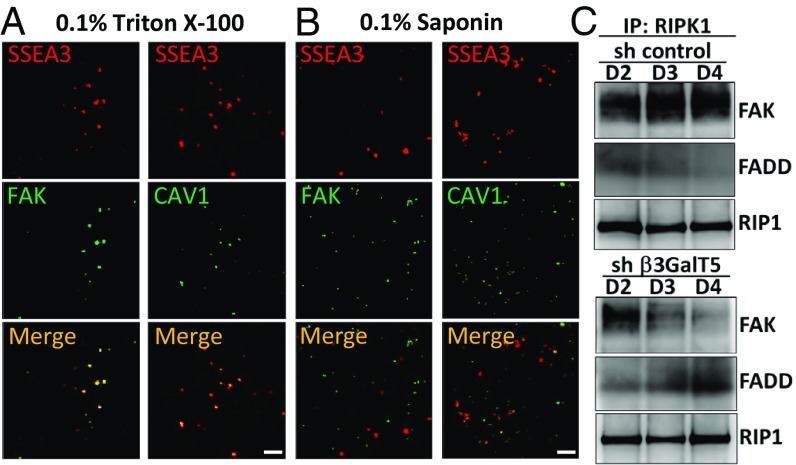
To examine whether CAV1 would form a complex with SSEA3/SSEA4 and FAK in breast cancer cells, we analyzed the colocalization of SSEA3/SSEA4, FAK, and CAV1 in MDA-MB-231 cells by immunofluorescence microscopy (SI Appendix, Fig. S6A) and stimulated emission depletion (STED) (Fig. 4A) after triton X-100 or saponin treatment. The results indicated that SSEA3, FAK, and CAV1 were strongly colocalized in the cytoplasm and the membrane raft of MDA-MB-231 cells because after treatment with saponin, a detergent used for the disassembly of membrane rafts, we found that neither SSEA3-FAK nor SSEA3-CAV1 was colocalized in membrane raft (Fig. 4B). To investigate whether SSEA3-FAK-CAV1 formed a complex in membrane rafts, we examined the expression of FAK and CAV1 in MDA-MB-231 cell lysates with or without SSEA3 (SI Appendix, Fig. S6B). We isolated the membrane raft fraction from cell lysate and verified by cholera toxin, which binds to the membrane raft marker GM1 on TLC-blot, and found that SSEA3 was enriched in the membrane raft. We also observed the existence of FAK and CAV1 in the lipid raft fraction and a decreased expression of FAK and CAV1 in β3GalT5 knockdown MDA-MB-231 cells. These results suggested that the SSEA3-CAV1-FAK complex may regulate the signaling in breast cancer cells.
Fig. 4.
Colocalization of SSEA3-FAK-CAV1 in MDA-MB-231cells. (A) The STED microscopy images of cells were obtained with antibody-conjugated fluorescent dye after 0.1% triton X-100 permeabilization in MDA-MB-231 cells. (B) Disruption of the SSEA3, CAV1, and FAK complex by lipid raft-sensitive detergent. The STED microscopy images in MDA-MB-231 cells were determined by antibody-conjugated fluorescent dye after 0.1% saponin permeabilization in MDA-MB-231 cells. SSEA3 is labeled with AF546 (red), and FAK and CAV1 are labeled with AF488 (green). The yellow color in merge panels indicates colocalization of SSEA3 and FAK or CAV1. (Scale bar, 2 μm.) (C) β3GalT5 regulates cell apoptosis through RIP shuttling from FAK to FADD. The cell lysate of MDA-MB-231 cells with knockdown of β3GalT5 or control were pulled down by anti-RIP antibody and analyzed by Western blot to determine the expression level of FAK and FADD, with the expression level of RIP as control.
Knockdown of β3GalT5 Caused RIP Association with FADD to Induce Cell Apoptosis.
It is shown that FAK would suppress apoptosis by binding to the death domain of RIP, which shuttles between the apoptosis and survival signaling pathways (22). To further investigate whether the apoptosis caused by the knockdown of β3GalT5 was through the dissociation of RIP from FAK to induce FADD-mediated apoptotic signaling, we analyzed the expression of FAK and FADD using anti-RIP antibody to pull down the RIP-binders from MDA-MB-231 cell lysate. The result showed that knocking down the expression of β3GalT5 reduced the interaction of RIP and FAK and enhanced the interaction of RIP and FADD (Fig. 4C), indicating that the SSEA3-FAK-CAV1 complex may regulate breast cancer survival via association with RIP to prevent its interaction with FADD for signaling.
Attenuation of Globo-Series Glycans Exposure Inhibited Tumor Growth.
Our previous study showed that breast cancer cells treated with FK506, an inhibitor of FK506-binding protein 4 (FKBP4), showed a decrease in SSEA4 expression on cell surface (24). To further investigate whether reducing the SSEA4 expression would induce cell apoptosis, MDA-MB-231 cells were treated with FK506 and caspase-3 inhibitor. It was found that cells treated with FK506 had lower SSEA4 expression on the surface and increased apoptosis (SI Appendix, Fig. S7 A–C), whereas treatment with caspase-3 inhibitor could rescue cell viability, suggesting that the external globo-series GSLs regulate the internal downstream signaling in breast cancer progression.
To identify additional binding proteins of the globo-series GSLs, we mixed cell lysates with biotinylated SSEA3, SSEA4, or Globo-H and pulled out the binders with neutravidin agarose beads for analysis by mass spectrometry. Using this affinity capture technique, we successfully identified the SSEA4 binding protein galectin-8 in breast cancer cells. By determining the glycan-binding profiles of galectin-8 and its N- and C-terminal carbohydrate recognition domains (N-CRD and C-CRD), we found that the NeuAcα2,3–β-galactoside motif was critical for galectin-8 binding to SSEA4 via N-CRD, and the C-CRD was highly selective for poly-LacNAc extension (SI Appendix, Fig. S8). Furthermore, knockdown of galectin-8 expression was found to dramatically increase the expression of SSEA4 on breast cancer cells (SI Appendix, Fig. S9) and also to enhance the cell migration (SI Appendix, Fig. S10 A and B) and invasion ability (SI Appendix, Fig. S10 C and D). These results suggested that galectin-8 plays a critical role in regulating SSEA4 trafficking and breast cancer transformation.
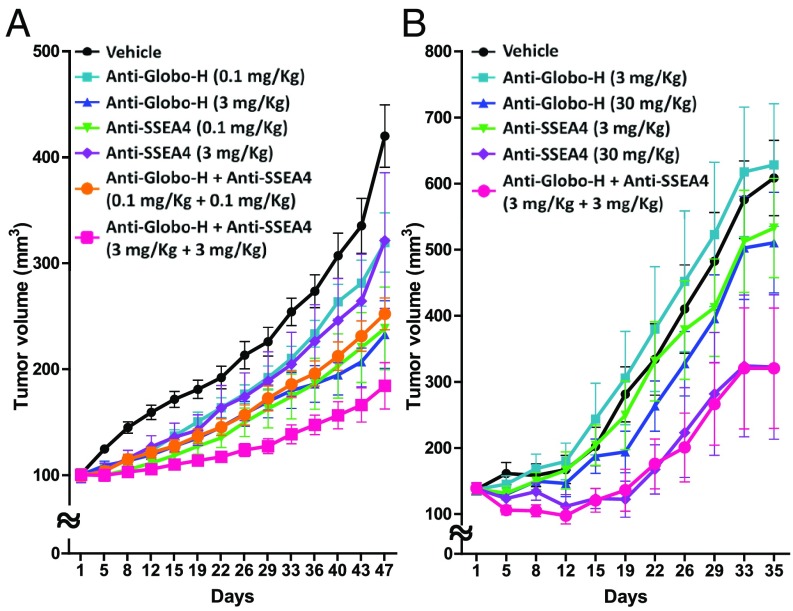
Interestingly, we also found that the tumor growth in mice xenografted with MCF7 was inhibited by treatment with anti-Globo-H and anti-SSEA4 monoclonal antibodies (3 mg/kg), with 45% and 24% of tumor growth inhibition (TGI), respectively, and 56% of TGI in combination treatment (Fig. 5A and SI Appendix, Fig. S11A). The synergistic effect was observed in the low-dose antibody combination treatment in HCC1428 (3 mg/kg each antibody) (Fig. 5B and SI Appendix, Fig. S11B) and pancreatic cancer HPAC (0.1 mg/kg each antibody) with 35% of TGI and 37% of TGI (SI Appendix, Fig. S11C). Targeting both SSEA4 and Globo-H simultaneously by the respective monoclonal antibodies resulted in an apparently synergistic tumor suppression, indicating that SSEA4 and Globo-H may play a parallel role to regulate tumor growth.
Fig. 5.
Synergistic or additive effect observed in combination of anti-Globo-H antibody and anti-SSEA4 antibody in MCF7- and HCC1428-implanted female nude mice. (A) Anti-Globo-H antibody (0.1 and 3 mg/kg, IV) or anti-SSEA4 (0.1 and 3 mg/kg, IV) antibody as single agent was active against breast cancer MCF7 xenograft, and the combination of both antibodies (3 + 3 mg/kg, IV) showed a greater antitumor activity (56% of TGI) compared with individual antibodies. (B) Anti-Globo-H antibody (3 and 30 mg/kg, IV) or anti-SSEA4 (3 and 30 mg/kg, IV) antibody as single agent was active against HCC1428 xenograft, and the combination of both antibodies (3 + 3 mg/kg, IV) showed a greater antitumor activity (35% of TGI) compared with individual antibodies. The tumor volume in each group (n = 8) was measured at different time points and is shown as mean ± SD. P < 0.0001 was determined by two-way RM ANOVA.
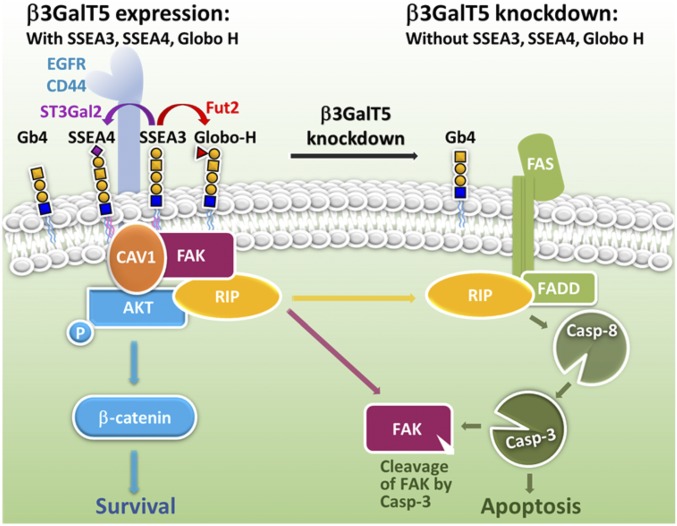
This study concluded that knockdown of β3GalT5 in breast cancer cells would suppress the expression of SSEA3/SSEA4/Globo-H complex (the globo-series GSL complex) on the cell surface and lead to the dissociation of RIP from the FAK/CAV1/AKT/RIP complex (the FAK complex) to interact with FADD for caspase-8 and -3 activation, leading to cell apoptosis and dysfunction of FAK (Fig. 6). The pivotal role of β3GalT5 and the globo-series GSLs in breast cancer cells and the cooperation of the globo-series GSLs with the FAK complex to suppress apoptosis and enhance malignant properties revealed in this study provide a better understanding of the globo-series GSL signaling in breast cancer and its application to cancer therapy as demonstrated by the combined use of antibodies against SSEA4 and Globo-H in this study and the Globo-H vaccine reported previously (1).
Fig. 6.
The critical roles of β3GalT5 and the globo-series GSLs in regulating the apoptosis and survival of breast carcinoma cells. A schematic diagram suggesting that in the absence of β3GalT5, the expressions of SSEA3, SSEA4, and Globo-H are down-regulated, leading to the dissociation of RIP from the FAK complex. The released RIP is then associated with FADD to facilitate the FAS-mediated cell apoptosis through caspase-8 and -3 activation and FAK degradation. On the contrary, in the presence of β3GalT5, SSEA3, SSEA4, and Globo-H are up-regulated and associated with CAV1/FAK/AKT/RIP to form a complex on membrane microdomain and prevent the activation of caspase-3 leading to breast carcinoma cell survival and metastasis. As indicated in the experiment, SSEA3/SSEA4 is more associated with CAV1, while SSEA3/Globo-H is more associated with FAK.
Discussion
Since hematopoietic or mesenchymal stem cells usually do not express SSEA3, so SSEA3 is not considered as an appropriate marker of multipotent cells (25). However, knockdown of β3GalT5 in this study was found to cause a significant down-regulation of the globo-series GSLs in MDA-MB-231 (SI Appendix, Fig. S2). This finding is consistent with the report that overexpression of globotriaosylceramide synthase (GCS) significantly enhanced the expression of Gb3, Gb4, SSEA3, and Globo-H in GEM and increased FAK-mediated beta-catenin activation to maintain tumorigenicity and multiple drug resistance in breast cancer stem cells (26). In addition, the N-terminal lipid-binding domain is required for the regulation of FAK translocated to membranes (27). These studies also indicated that the globo-series GSLs and the FAK complex are contributed to the up-regulation of CAV1 expression for migration enhancement during epithelial to mesenchymal transition (EMT) (28). We have also found that the antibodies which are currently available and specific for the globo-series GSLs could not differentiate the isomers with α1,3- and α1,4-linkage between the two Gal residues, and the expression level of SSEA4 was increased in tyrosine kinase inhibitor (TKI)-resistant nonsmall cell lung cancer cell lines such as H1975 (L858R/T790M). It was reported that siglec-7 and -9 on NK cells could interact with α2,3- or α2,6-linked sialosides on cancer cells, and, as a result, the NK cell was negatively regulated (29). It would be interesting to understand the role of SSEA4 in the drug-resistant cancer cells and whether SSEA4 and its sialylated derivatives (e.g., α2,8-linked sialylation on the sialic acid residue, or α2,6-linked sialylation on the GalNAc residue) on cancer cells would interact with NK cells and other immune cells to inhibit their response to cancer cells.
The transmembrane protein CAV1 is known to cooperate with cell surface receptors and lipid raft for regulating the prognosis status, including poor survival rate, metastasis, and multidrug resistance (30). Palmitic acid (C16:0 fatty acids) at the C terminus of CAV1 enhances the clustering of GSLs in GEM and provides a platform for protein–protein or protein–lipid interaction (31). Interestingly, from the pulldown analysis of SSEA3 in MDA-MB-231 and MCF7 cell lysates, only the C16:0 fatty acid was detected by LC-MS/MS, suggesting a possible interaction of CAV1 with GSLs through C16:0. Further evidence showed that disruption of the raft composition led to FAK down-regulation, anoikis-like apoptosis, and depletion of caveolae formation to restrict CAV1 exposure (32), indicating that attenuation of FAK caused the limitation of CAV1 exposure and resulted in the accumulation of SSEA3 inside the cell.
In addition, overexpression of the CAV1 mutant with no palmitoylation in cancer cells inhibits AKT, suggesting that palmitoylation of CAV1 is important to activate AKT signaling to promote tumor growth and migration (33). AKT modulated tumor growth by interacting with cell surface receptors, including epidermal growth factor receptor (EGFR) and CD44, which were abundant in basal-like breast cancer and displayed on cell surface, and inhibition of FAK and EGFR interaction led to cell detachment and apoptosis through caspase-3 dependent degradation of AKT and FAK (34). Moreover, it was found that AKT was directly associated with FAK (23) and RIP kinase (35) to escape cell death and stimulate metastasis, supporting the notion that formation of the RIP-FAK-AKT complex is required for tumor progression. Recent studies also showed that knockdown of CD44 in cancer-initiated cells suppressed the proliferative and metastatic potential via reduction of AKT and FAK phosphorylation, indicating that CD44 exhibited its influence on carcinogenesis via regulation of FAK and the downstream AKT/beta-catenin signaling pathway (36).
In summary, our findings revealed the interaction of globo-series GSLs with the FAK complex, and this interaction was shown to play an essential role in cancer proliferation and metastasis through the downstream AKT survival signaling pathways. In addition, intervention or disruption of this globo-series GSL signaling was shown to be an effective anticancer strategy as demonstrated in the use of carbohydrate-based antibodies or vaccines to target the globo-series glycans on cancer cells.
Materials and Methods
For β3GalT5 staining, tissue array slides comprising a total of 39 normal breast sections and 142 breast carcinoma sections were taken from the tissues of 152 patients. Paraffin-embedded tissue blocks were collected from Wan Fang Hospital managed by Taipei Medical University Hospital (Taiwan). Patient information, including gender, age, and histopathological diagnoses, was collected. Follow-up of patients was carried out for up to 151 mo and approval of the Institutional Review Boards and with permission from the ethics committees of the Taipei Medical University Hospital (TMU-IRB 99049).
For additional details, see SI Appendix, Materials and Methods.
Supplementary Material
Acknowledgments
We thank our colleagues at Academia Sinica, Dr. Tsung-Ching Lai and Ms. Hsing-Fang Tsai, for experimental assistance of IHC study and supply of various cancer cell lines; Dr. Fu-Tong Liu for all human galectins primers in qPCR assay; Dr. Chien-Tai Ren for the poly-LacNAc glycans; the RNAi core for human galectin-8 shRNA design and lenti-CRISPR/Cas9 vector construction; and the glycan sequencing and mass spectrometry core facilities at the Genomics Research Center, for glycolipid analysis. This research was supported by the Summit Program of the Genomics Research Center, Academia Sinica, Taiwan.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1816946116/-/DCSupplemental.
References
- 1.Huang Y-L, et al. Carbohydrate-based vaccines with a glycolipid adjuvant for breast cancer. Proc Natl Acad Sci USA. 2013;110:2517–2522. doi: 10.1073/pnas.1222649110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang W-W, et al. Expression of Globo H and SSEA3 in breast cancer stem cells and the involvement of fucosyl transferases 1 and 2 in Globo H synthesis. Proc Natl Acad Sci USA. 2008;105:11667–11672. doi: 10.1073/pnas.0804979105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lou Y-W, et al. Stage-specific embryonic antigen-4 as a potential therapeutic target in glioblastoma multiforme and other cancers. Proc Natl Acad Sci USA. 2014;111:2482–2487. doi: 10.1073/pnas.1400283111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheung SKC, et al. Stage-specific embryonic antigen-3 (SSEA-3) and β3GalT5 are cancer specific and significant markers for breast cancer stem cells. Proc Natl Acad Sci USA. 2016;113:960–965. doi: 10.1073/pnas.1522602113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou D, Henion TR, Jungalwala FB, Berger EG, Hennet T. The beta 1,3-galactosyltransferase beta 3GalT-V is a stage-specific embryonic antigen-3 (SSEA-3) synthase. J Biol Chem. 2000;275:22631–22634. doi: 10.1074/jbc.C000263200. [DOI] [PubMed] [Google Scholar]
- 6.Saito S, et al. Human alpha2,3-sialyltransferase (ST3Gal II) is a stage-specific embryonic antigen-4 synthase. J Biol Chem. 2003;278:26474–26479. doi: 10.1074/jbc.M213223200. [DOI] [PubMed] [Google Scholar]
- 7.Rajan VP, Larsen RD, Ajmera S, Ernst LK, Lowe JB. A cloned human DNA restriction fragment determines expression of a GDP-L-fucose: Beta-D-galactoside 2-alpha-L-fucosyltransferase in transfected cells. Evidence for isolation and transfer of the human H blood group locus. J Biol Chem. 1989;264:11158–11167. [PubMed] [Google Scholar]
- 8.Rouquier S, et al. Molecular cloning of a human genomic region containing the H blood group alpha(1,2)fucosyltransferase gene and two H locus-related DNA restriction fragments. Isolation of a candidate for the human secretor blood group locus. J Biol Chem. 1995;270:4632–4639. doi: 10.1074/jbc.270.9.4632. [DOI] [PubMed] [Google Scholar]
- 9.Levina V, Marrangoni AM, DeMarco R, Gorelik E, Lokshin AE. Drug-selected human lung cancer stem cells: Cytokine network, tumorigenic and metastatic properties. PLoS One. 2008;3:e3077. doi: 10.1371/journal.pone.0003077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki Y, et al. SSEA-3 as a novel amplifying cancer cell surface marker in colorectal cancers. Int J Oncol. 2013;42:161–167. doi: 10.3892/ijo.2012.1713. [DOI] [PubMed] [Google Scholar]
- 11.Seko A, et al. Beta1,3-galactosyltransferases-4/5 are novel tumor markers for gynecological cancers. Tumour Biol. 2009;30:43–50. doi: 10.1159/000203129. [DOI] [PubMed] [Google Scholar]
- 12.Kang X, et al. Glycan-related gene expression signatures in human metastatic hepatocellular carcinoma cells. Exp Ther Med. 2012;3:415–422. doi: 10.3892/etm.2011.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo Z, et al. Side population in hepatocellular carcinoma HCCLM3 cells is enriched with stem-like cancer cells. Oncol Lett. 2016;11:3145–3151. doi: 10.3892/ol.2016.4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steelant WF, et al. Monosialyl-Gb5 organized with cSrc and FAK in GEM of human breast carcinoma MCF-7 cells defines their invasive properties. FEBS Lett. 2002;531:93–98. doi: 10.1016/s0014-5793(02)03484-1. [DOI] [PubMed] [Google Scholar]
- 15.Van Slambrouck S, Steelant WF. Clustering of monosialyl-Gb5 initiates downstream signalling events leading to invasion of MCF-7 breast cancer cells. Biochem J. 2007;401:689–699. doi: 10.1042/BJ20060944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Owens LV, et al. Overexpression of the focal adhesion kinase (p125FAK) in invasive human tumors. Cancer Res. 1995;55:2752–2755. [PubMed] [Google Scholar]
- 17.Sulzmaier FJ, Jean C, Schlaepfer DD. FAK in cancer: Mechanistic findings and clinical applications. Nat Rev Cancer. 2014;14:598–610. doi: 10.1038/nrc3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golubovskaya VM, Zheng M, Zhang L, Li J-L, Cance WG. The direct effect of focal adhesion kinase (FAK), dominant-negative FAK, FAK-CD and FAK siRNA on gene expression and human MCF-7 breast cancer cell tumorigenesis. BMC Cancer. 2009;9:280. doi: 10.1186/1471-2407-9-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu LH, et al. The focal adhesion kinase suppresses transformation-associated, anchorage-independent apoptosis in human breast cancer cells. Involvement of death receptor-related signaling pathways. J Biol Chem. 2000;275:30597–30604. doi: 10.1074/jbc.M910027199. [DOI] [PubMed] [Google Scholar]
- 20.Wen LP, et al. Cleavage of focal adhesion kinase by caspases during apoptosis. J Biol Chem. 1997;272:26056–26061. doi: 10.1074/jbc.272.41.26056. [DOI] [PubMed] [Google Scholar]
- 21.Kurenova E, et al. Focal adhesion kinase suppresses apoptosis by binding to the death domain of receptor-interacting protein. Mol Cell Biol. 2004;24:4361–4371. doi: 10.1128/MCB.24.10.4361-4371.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamarajan P, Bunek J, Lin Y, Nunez G, Kapila YL. Receptor-interacting protein shuttles between cell death and survival signaling pathways. Mol Biol Cell. 2010;21:481–488. doi: 10.1091/mbc.E09-06-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang S, Basson MD. Akt directly regulates focal adhesion kinase through association and serine phosphorylation: Implication for pressure-induced colon cancer metastasis. Am J Physiol Cell Physiol. 2011;300:C657–C670. doi: 10.1152/ajpcell.00377.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hung T-C, Lin C-W, Hsu T-L, Wu C-Y, Wong C-H. Investigation of SSEA-4 binding protein in breast cancer cells. J Am Chem Soc. 2013;135:5934–5937. doi: 10.1021/ja312210c. [DOI] [PubMed] [Google Scholar]
- 25.Suila H, et al. Are globoseries glycosphingolipids SSEA-3 and -4 markers for stem cells derived from human umbilical cord blood? J Mol Cell Biol. 2011;3:99–107. doi: 10.1093/jmcb/mjq041. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, et al. Glucosylceramide synthase upregulates MDR1 expression in the regulation of cancer drug resistance through cSrc and beta-catenin signaling. Mol Cancer. 2010;9:145. doi: 10.1186/1476-4598-9-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leonard TA, Hurley JH. Regulation of protein kinases by lipids. Curr Opin Struct Biol. 2011;21:785–791. doi: 10.1016/j.sbi.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bailey KM, Liu J. Caveolin-1 up-regulation during epithelial to mesenchymal transition is mediated by focal adhesion kinase. J Biol Chem. 2008;283:13714–13724. doi: 10.1074/jbc.M709329200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.RodrÍguez E, Schetters STT, van Kooyk Y. The tumour glyco-code as a novel immune checkpoint for immunotherapy. Nat Rev Immunol. 2018;18:204–211. doi: 10.1038/nri.2018.3. [DOI] [PubMed] [Google Scholar]
- 30.Martinez-Outschoorn UE, Sotgia F, Lisanti MP. Caveolae and signalling in cancer. Nat Rev Cancer. 2015;15:225–237. doi: 10.1038/nrc3915. [DOI] [PubMed] [Google Scholar]
- 31.Di Vizio D, et al. Caveolin-1 interacts with a lipid raft-associated population of fatty acid synthase. Cell Cycle. 2008;7:2257–2267. doi: 10.4161/cc.7.14.6475. [DOI] [PubMed] [Google Scholar]
- 32.Park E-K, et al. Cholesterol depletion induces anoikis-like apoptosis via FAK down-regulation and caveolae internalization. J Pathol. 2009;218:337–349. doi: 10.1002/path.2531. [DOI] [PubMed] [Google Scholar]
- 33.Mollinedo F, Gajate C. Lipid rafts as major platforms for signaling regulation in cancer. Adv Biol Regul. 2015;57:130–146. doi: 10.1016/j.jbior.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 34.Golubovskaya V, et al. Dual inhibition of focal adhesion kinase and epidermal growth factor receptor pathways cooperatively induces death receptor-mediated apoptosis in human breast cancer cells. J Biol Chem. 2002;277:38978–38987. doi: 10.1074/jbc.M205002200. [DOI] [PubMed] [Google Scholar]
- 35.Liu Q, et al. Akt and mTOR mediate programmed necrosis in neurons. Cell Death Dis. 2014;5:e1084. doi: 10.1038/cddis.2014.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nam K, Oh S, Lee KM, Yoo SA, Shin I. CD44 regulates cell proliferation, migration, and invasion via modulation of c-Src transcription in human breast cancer cells. Cell Signal. 2015;27:1882–1894. doi: 10.1016/j.cellsig.2015.05.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.