Abstract
Background
Zoledronate has anti-bone resorption activity and is reported to reduce skeletal-related events. The objective of this study was to test the hypothesis that addition of zoledronate in chemotherapy is safe and effective in osteosarcoma.
Material/Methods
A total of 798 patients, age 25 years and above, with newly diagnosed high-grade surgically salvageable malignant osteosarcoma, were included in the trial. All patients had received standard chemotherapies (n=399). In addition, in a standard chemotherapy regimen, patients enrolled in the zoledronate group also received 10 courses of 4 mg intravenous infusions of zoledronate (n=399). Limb-sparing surgery was performed by orthopedic surgeons (n=798). Clinical assessment, laboratory monitoring, overall survival, event-free survival, and treatment-emergent adverse effects were evaluated. The chi-square independence-samples test was used for statistical analysis at 95% confidence level.
Results
The histopathological response was the same for both groups (p=0.12). Addition of zoledronate to chemotherapy improved skeletal event-free survival (p=0.04) but decreased overall survival (p=0.02). Zoledronate induced hypocalcemia (p<0.0001), hypophosphatemia (p<0.0001), cardiotoxicity (p<0.0001), lung metastases (p=0.03), flu-like syndrome (p<0.0001), and ototoxicity (p=0.02), and elevated serum aspartate aminotransferase (p<0.0001) and serum alanine aminotransferase (p<0.0001).
Conclusions
The addition of zoledronate to standard chemotherapy in high-grade resectable osteosarcoma is detrimental and is not advised.
MeSH Keywords: Antineoplastic Combined Chemotherapy Protocols, Disease-Free Survival, Neoplasm Metastasis, Osteosarcoma, Survival Analysis, Survival Rate
Background
Osteosarcoma is a malignant tumor of the bone [1]. In general practice, it is treated with chemotherapeutic agents [2]. Etoposide, doxorubicin, methotrexate, ifosfamide, and cisplatin have been found effective in various stages of osteosarcoma [1]. The overall survival after a relapse and/or with metastases in osteosarcoma is low [3]. Therefore, new osteosarcoma drugs are required to improve outcome and to decrease treatment-emergent toxic effects.
Bisphosphonates are widely used to treat tumor-induced osteolysis-related bone problems of patients [2] and also can inhibit osteoclastic bone resorption [3]. Bisphosphonates inhibit the adhesion of cells of the tumor to the bone matrix [4]. Among bisphosphonates, zoledronate or zoledronic acid (third generation bisphosphonate) is approved in adults with solid tumors [5], has the highest anti-bone resorption activity, and also shows a reduction in skeletal-related events in cancer [6]. It also was reported to have anti-tumor activity and inhibits cancer cell migration [2]. A study demonstrated that a combination of chemotherapy with zoledronic acid in a predominately white population of osteosarcoma patients treated with surgery does not improve skeletal-related events-free survival, but increased the chances of death [3]. However, another study reported that zoledronic acid can be safely added to chemotherapy for osteosarcoma patients [7]. At the same time as the other randomized study, we performed a phase III trial on zoledronate with chemotherapy to assess its efficacy and safety in patients with osteosarcoma patients treated with limb-sparing surgery in an older Asian population.
The primary aim of this study was to test the hypothesis that addition of zoledronate to the standard chemotherapy is effective and safe in osteosarcoma patients treated with limb-sparing surgeries. The secondary endpoint of the study was to compare skeletal-related events-free survival and overall survival for zoledronate and chemotherapy combined with chemotherapy alone at the level I of evidence without conflict of interest.
Material and Methods
Drugs
Methotrexate was purchased from Medac Pharma, Inc., USA. Etoposide was purchased from Pfizer Inc., USA. Ifosfamide (Ifex®) was purchased from Bristol-Myers Squibb Co. New York, USA. Doxorubicin (Adriamycin®) was purchased from Pfizer, Inc., USA. Cisplatin (Platinol®) was purchased from Bristol-Myers Squibb Co., New York, USA. Zoledronate (Zometa®) was purchased from Beijing Novartis Pharma Co., Beijing, China. Calcium and 25-Hydroxyvitamin D tablets were purchased from Glenmark Pharmaceuticals, India. Normal saline was purchased from Shanghai Baxter Healthcare Co., Shanghai, China.
Ethical approval and consent to participate
The study was registered with the Research registry (www.researchregistry.com), UID No. researchregistry4414 dated 1 September 2009. The protocol of the study (CMU/CL/3/09 dated 15 August 2009) was approved by the Cancer Hospital of China Medical University review board. The study adhered to the law of China, consolidated standards of reporting trials (CONSORT), and the Declaration of Helsinki (V2008). The trial was reported in line with the Strengthening the Reporting of Cohort Studies in Surgery (STROCSS) criteria [8]. All patients signed an informed consent form for interventions, pathology, surgeries, and publication(s) of the study in all formats (hard and/or electronics) including patients’ personal information (image or photograph if any) irrespective of time and language.
Inclusion criteria
We enrolled patients age 25 years and above with newly diagnosed high-grade surgically salvageable malignant osteosarcoma confirmed by biopsies admitted to the Cancer Hospital of China Medical University, China and the referring hospitals from 15 September 2011 to 1 August 2013. The demographic characteristics of the enrolled patients are presented in Table 1.
Table 1.
Demographical characteristics of the patients.
| Characteristics | Groups | Comparison between groups | ||
|---|---|---|---|---|
| CL | ZA | |||
| Numbers of patients | 399 | 399 | ||
| Intervention | Standard chemotherapy only | Standard chemotherapy with zoledronate | p-Value | |
| Gender | Male | 230 (58) | 207 (52) | 0.12 |
| Female | 169 (42) | 192 (48) | ||
| Age (years) | Maximum | 59 | 61 | 0.09 |
| Minimum | 26 | 26 | ||
| Mean ±SD | 31.45±5.45 | 32.27±7.85 | ||
| Between 25–40 years | 150 (38) | 150 (38) | ||
| Anatomical tumor site | Axial | 21 (5) | 18 (4) | 0.74 |
| Limb | 378 (95) | 381 (96) | ||
| Tumor size* | <10 mm | 221 (55) | 236 (59) | 0.32 |
| ≥10 mm | 178 (45) | 163 (41) | ||
| Histological subtype osteosarcoma | Small cell | 7 (2) | 3 (1) | 0.07 |
| Convectional | 374 (391) | 371 (92) | ||
| Telangiectasic | 3 (1) | 4 (1) | ||
| High-grade surface | 4 (1) | 3 (1) | ||
| Giant cell | 4 (1) | 8 (2) | ||
| well-differentiated | 3 (1) | 4 (1) | ||
| Juxtacortical | 4 (1) | 3 (1) | ||
| Pleomorphic bone | 0 (0) | 3 (1) | ||
| Fibroma-chondromyxoid | 8 (2) | 0 (0) | ||
| Pathologically fracture | Yes | 38 (10) | 29 (7) | 0.31 |
| No | 361 (90) | 370 (93) | ||
| Staging | Localized | 282 (71) | 267 (67) | 0.07 |
| Possible metastases | 86 (22) | 81 (20) | ||
| Definite metastases | 31 (7) | 51 (13) | ||
| Staging according to American joint committee on cancer system for osteosarcoma# | I-A | 85 (21) | 80 (20) | 0.33 |
| I-B | 115 (29) | 131 (33) | ||
| II-A | 48 (12) | 21 (5) | ||
| II-B | 95 (24) | 99 (25) | ||
| III | 25 (6) | 17 (4) | ||
| IV-A | 20 (5) | 36 (9) | ||
| IV-B | 11 (3) | 15 (4) | ||
Data were represented as a number (percentage) and mean ±SD for constant and continuous data respectively. For statistical analysis, the Chi-square Independence test was used for constant data and the two-tailed paired test was used for continuous data. A p<0.01 was considered significant.
The largest diameter.
All patients were from P.R. China.
I – Low and no-metastasis; II – high and no-metastasis; III – any with pulmonary metastasis; IV – any with non-pulmonary metastasis; A – <8 cm; B – >8 cm.
Exclusion criteria
Patients age below 25 years, with abnormal renal functions (the estimated glomerular filtration rate <70 mL/min/m2), liver functions (bilirubin dosage higher than twice), and cardiac functions (ventricular ejection fraction <50%) were excluded from the trial. Patients who previously received standard chemotherapies were excluded from the study. Patients with small cell osteosarcoma, maxillary osteonecrosis, osteosarcoma of the jaw, history of severe dental events, and surgically unsalvageable malignant osteosarcoma were excluded from the trial.
Experimental design
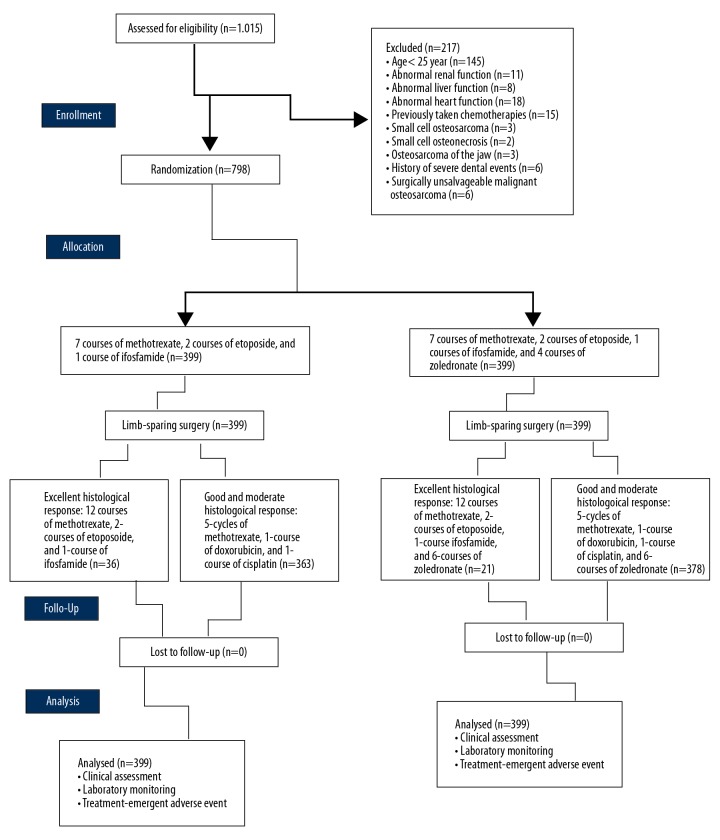
A total of 798 patients who signed an informed consent form were subjected to 1: 1 randomization. Blinding was carried out by prefilled envelopes and maintained throughout the trial by the Cancer Hospital of China Medical University, China itself. The physicians involved in the randomization were not involved in the treatment. The sample size was calculated by PASS 16.0.3, NCSS, LLC, Utah, USA. The sample size was found to be 399 for both groups. Confidence intervals for both sides was 95% (α=0.05). A CONSORT flow diagram of the study is presented in Figure 1.
Figure 1.
CONSORT flowchart of the trial. Confidence intervals for both sides was 95% (α=0.05).
Preoperative intervention
Before surgeries, all enrolled patients received 7 courses of 12 g/m2 methotrexate, 2 courses of 300 mg/m2 etoposide, and 1 course of 12 g/m2 ifosfamide. Two courses of the same kind had 21 days of recovery period (they were given every 3 weeks) [3]. In addition to a chemotherapy regimen, patients enrolled in the zoledronate group (ZA group) also received 4 courses of 4 mg intravenous infusions of zoledronate/month [9].
Limb-sparing surgery
All enrolled patients were subjected to limb-sparing surgery after preoperative chemotherapies by an orthopedic surgeon (blinded regarding randomization; minimum 3 years of experience). During surgeries, the cancerous tissue and surrounding healthy tissues were removed. Limb-sparing surgery was performed by 7 different orthopedic surgeons of the Cancer Hospital of China Medical University, China [10]. Resected bone tumors were subjected to histopathological analysis. Grade was rated as excellent (more than 90% necrosis of tumor), good (60–90% necrosis of tumor), and moderate (40–60% necrosis of tumor).
Postoperative intervention
After surgeries, patients with excellent histological response and without metastasis received 12 courses of 12 g/m2 methotrexate, 2 courses of 300 mg/m2 etoposide, and 1 course of 12 g/m2 ifosfamide. Patients with good and moderate histological response, salvageable malignant osteosarcoma, and/or initial metastases received 5 cycles of 12 g/m2 methotrexate, 1 course of 75 mg/m2 doxorubicin, and 1 course of 120 mg/m2 cisplatin. Two courses of the same kind had 21 days of recovery period [3]. In addition to a chemotherapy regimen, patients enrolled in the ZA group also received 6 courses of 4-mg intravenous infusions of zoledronate per month [9].
Both groups of patients received 500 mg calcium [11] with 400 IU 25-hydroxy Vitamin D/day [12] during the trial and in the follow-up period (as per requirements).
Clinical assessment
Clinical assessment was performed during chemotherapy regimens at 7 weeks and 14 weeks with X-ray and magnetic resonance imaging (MRI) for the tumor site and the chest. After completion of the regimen, a yearly X-ray assessment of the tumor site and the chest was performed. Event-free survival was considered as the time until the development of relapse or secondary tumor at the site of limb and/or lung(s) from the time of enrollment. Overall survival was considered as from the time of detection of osteosarcoma to death [3]. Only 1 radiographic center of the Cancer Hospital of China Medical University, China and its staff (blinded regarding interventions) was involved in the clinical assessments.
Laboratory monitoring
Hematological, hepatic, and renal functions were monitored. Serum calcium level and serum phosphate level were measured at 1 day and 3 days, then once a week after the first zoledronate course, and before each infusion and 2 days after infusion for the subsequent courses. Serum aspartate aminotransferase level, serum alanine aminotransferase level, serum creatinine level, and serum bilirubin level were evaluated after each cycle. Less than 6.40 mg/dL serum calcium level was considered as hypocalcemia and less than 2.50 mg/dL serum phosphate level was considered as hypophosphatemia. More than 80 unit/L and more than 100 unit/L of serum aspartate aminotransferase and serum alanine aminotransferase were considered as an elevated level. More than 1.7 mg/dL for males and more than 1.4 mg/dL for females were considered as elevated serum creatinine level, and less than 0.3 mg/dL was considered as a normal level of serum bilirubin [3]. Only 1 pathological laboratory of the Cancer Hospital of China Medical University, China and its staff (blinded regarding interventions) was involved in the laboratory monitoring.
Treatment-emergent adverse event monitoring
Adverse events were checked after each cycle. Events were considered as adverse effects according to the v3.0-Common Terminology Criteria for Adverse Events (CTCAE; v3.0) [11]. Paramedical staff (blinded regarding interventions) of the Cancer Hospital of China Medical University, China were involved in the evaluation of adverse effects. Less than 1000 absolute neutrophil count/μL of blood was considered as neutropenia. Less than 13 g/L of blood for males and less than 12 g/L of blood for females hemoglobin count were considered as anemia [13]. Less than 50 000 platelet count/μL of blood was considered as thrombocytopenia. Cardiotoxicity 5–55% was defined as reduction in left ventricular ejection fraction, signs (tachycardia and/or S3 gallop), and symptoms of heart failure [14].
Statistical analysis
InStat, GraphPad Software, IL, USA was used for statistical analysis. The chi-square independent-samples test [3] was used for constant data and the two-tailed paired test was used for continuous data. An intention-to-treat method of analysis was used. All data were considered significant at 95% confidence level.
Results
The time required for chemotherapy before surgeries was the same between both groups (p=0.09). The time taken for surgeries after preoperative chemotherapies between groups (p=0.08) and the start of chemotherapy after surgeries (p=0.07) were the same in both groups.
Addition of zoledronate to chemotherapy improved event-free survival (p=0.04, Figure 2) but decreased overall survival (p=0.02, Figure 3). There was a clear trend for worse survival even in patients age 25–40 years in the ZA group (p=0.06, Figure 4). Overall survival for male patients was the same in both groups (p=0.74, Figure 5) but overall survival was reduced for female patients who received zoledronate in addition to chemotherapy (p=0.01, Figure 6).
Figure 2.
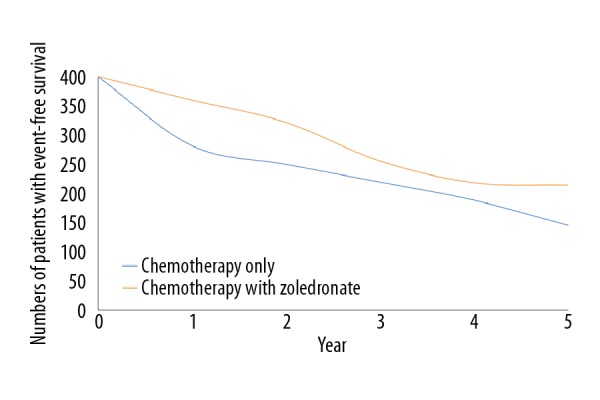
Event-free survival. We evaluated 399 patients in each group. The chi-square independent-samples test was used in statistical analysis. A p<0.05 was considered significant. 0: After completion of the regimen. Event-free survival was considered as the time of enrollment to development of relapse or secondary tumor.
Figure 3.
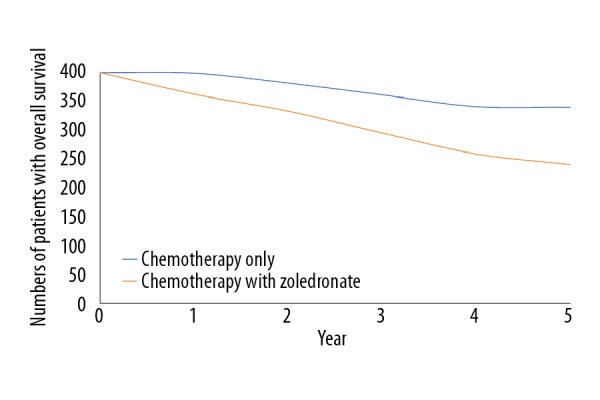
Overall survival of patients. We evaluated 399 patients in each group. The chi-square independent-samples test was used in statistical analysis. A p<0.05 was considered significant. 0: After completion of the regimen. Overall survival considered from the time of detection of osteosarcoma to death.
Figure 4.
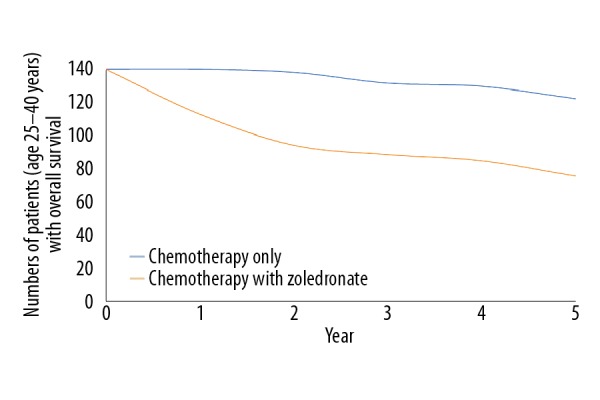
Overall survival of patients ages 25–40 years. We evaluated 150 patients in each group. The chi-square independent-samples test was used in statistical analysis. A p<0.05 was considered significant. 0: After completion of the regimen. Overall survival considered from the time of detection of osteosarcoma to death.
Figure 5.
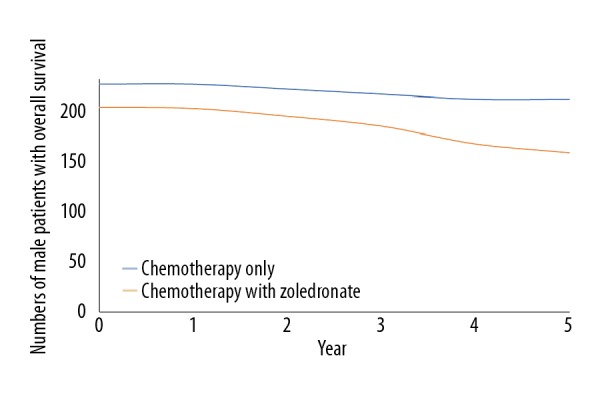
Overall survival of male patients. We evaluated 207 male patients for the chemotherapy with zoledronate group and 230 male patients for the chemotherapy alone group. The chi-square independent-samples test was used in statistical analysis. A p<0.05 was considered significant. 0: After completion of the regimen. Overall survival considered from time of detection of osteosarcoma to death.
Figure 6.
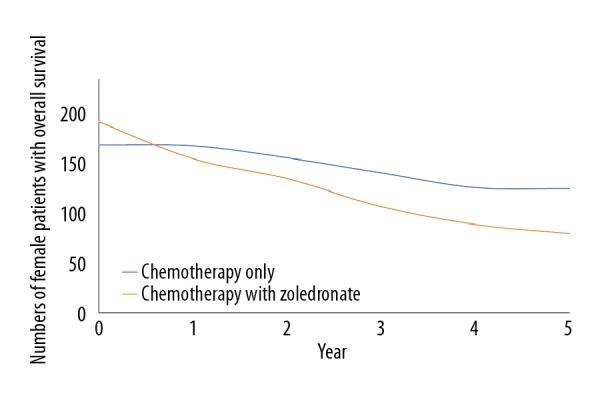
Overall survival of female patients. We evaluated 192 female patients for chemotherapy in the zoledronate group and 169 female patients for the chemotherapy alone group. The chi-square independent-samples test was used in statistical analysis. A p<0.05 was considered significant. 0: After completion of the regimen. Overall survival was considered as from the time of detection of osteosarcoma to death.
Patients who received chemotherapy with zoledronate had a higher number of lung metastases than in patients receiving chemotherapy alone (p=0.03). The histological response was the same for both groups (p=0.12). Zoledronate was responsible for hypocalcemia, hypophosphatemia, and elevation of serum aspartate aminotransferase and serum alanine aminotransferase (Table 2).
Table 2.
Laboratory monitoring.
| Characteristics | Groups | Comparison between groups | |
|---|---|---|---|
| CL | ZA | ||
| Numbers of patients | 399 | 399 | |
| Intervention | Standard chemotherapy only | Standard chemotherapy with zoledronate | p-Value |
| Hypophosphatemia | 26 (7) | 115 (29)* | <0.0001 |
| Serum aspartate aminotransferase elevation | 69 (17) | 170 (43)* | <0.0001 |
| Serum alanine aminotransferase elevation | 125 (31) | 220 (55)* | <0.0001 |
| Creatinine elevation | 138 (35) | 231 (58)* | <0.0001 |
| Bilirubin elevation | 149 (37) | 165 (41) | 0.28 |
| Histopathological response after completion preoperative Intervention | 167 (42) | 179 (45) | 0.43 |
| Excellent1 | 36 (9) | 21 (5) | 0.12 |
| Good2 | 254 (64) | 262 (66) | |
| Moderate3 | 109 (27) | 116 (29) | |
| Lung metastases# | 1 (0.25) | 9 (2)* | 0.03 |
Data were represented as number (percentage). The Chi-square Independence test was used in statistical analysis. A p<0.05 was considered significant. <6.40 mg/dL serum calcium level was considered as hypocalcemia and <2.50 mg/dL serum phosphate level was considered as hypophosphatemia. More than 80 unit/L and more than 100 unit/L were considered the elevated level of serum aspartate aminotransferase and serum alanine aminotransferase. >1.7 mg/dL for male and >1.4 mg/dL for female were considered as elevated serum creatinine. >0.3 mg/dL serum bilirubin was considered as the elevated level.
Significant zoledronate-emergent toxic effect.
Only one pathological laboratory of the Cancer Hospital of China Medical University, China and its staff (blinded regarding interventions) was involved.
X-ray and MRI results by a radiologist(s) of the Cancer Hospital of China Medical University, China.
– >90% necrosis of tumor;
– 60–90% necrosis of tumor;
– 40–60% necrosis of the tumor.
Zoledronate treatment has a high probability and high impact for treatment-emergent cardiotoxicity and fracture(s) (Table 3). Zoledronate treatment increased the risk for the treatment-emergent flu-like syndrome and ototoxicity. Patients did not die of complications related to fractures. The increased number of metastases did not alter the statistics, since the worse survival was not due to osteosarcoma. Zoledronate increased rates of cardiotoxicity and neutropenia, and was associated with a very slight increase in febrile neutropenia and an increase in postoperative wound healing complications. Patients died due to cardiotoxicities, multiple organ failures, and the other problems (Table 4).
Table 3.
Treatment-emergent toxic effect.
| Groups | Comparison between groups | |||||
|---|---|---|---|---|---|---|
| CL group | ZA group | |||||
| Numbers of patients | 399 | 399 | ||||
| Intervention | Standard chemotherapy only | Standard chemotherapy with zoledronate | For all grades | For severe toxicity | ||
| Adverse event | All grades | Severe toxicity | All grades | Severe toxicity | p-Value | p-Value |
| Neutropenia# | 317 (79) | 285 (71) | 338 (85) | 290 (73) | 0.06 | 0.75 |
| Anemia## | 354 (89) | 91 (23) | 370 (93) | 104 (26) | 0.07 | 0.32 |
| Thrombocytopenia### | 265 (66) | 81 (20) | 271 (68) | 92 (23) | 0.71 | 0.39 |
| Cardiotoxicity@ | 84 (21) | 11 (3) | 125 (31)& | 46 (12)* | 0.001 | <0.0001 |
| Fractures@@ | 26 (7) | 3 (1) | 92 (23)& | 32 (8)* | <0.0001 | <0.0001 |
<1000 absolute neutrophil count/μL blood;
<13 g/L for male and <12 g/L for female hemoglobin;
<50,000 platelet count count/μL blood.
5–55% reduction in left ventricular ejection fraction, signs (tachycardia and/or S3 gallop), and symptoms of heart failure Data were represented as number (percentage).
The Chi-square Independence test was used in statistical analysis. A p<0.05 was considered significant. As per CTCAE; v3.0. Paramedical staff (blinded regarding interventions) of the Cancer Hospital of China Medical University, China was involved in the evaluation of adverse effects.
Significant zoledronate-emergent severe toxicity.
Significant zoledronate-emergent toxic effect (all grade).
Most of female patients in both group and if male patient age >40 years.
Table 4.
Treatment-emergent adverse event.
| Adverse event | Groups | Comparison between groups | |
|---|---|---|---|
| CL group | ZA group | ||
| Numbers of patients | 399 | 399 | |
| Intervention | Standard chemotherapy only | Standard chemotherapy with zoledronate | p-Value |
| Weight loss | 241 (60) | 258 (65) | 0.24 |
| Flu-like syndrome (fever) | 170 (43) | 242 (61)* | <0.0001 |
| Mood disturbance | 30 (8) | 25 (6) | 0.58 |
| Headache | 27 (7) | 31 (8) | 0.68 |
| Ototoxicity | 124 (31) | 157 (39)* | 0.02 |
| Skin rashes | 131 (33) | 141 (35) | 0.51 |
| Postoperative wound healing complications | 15 (4) | 25 (6) | 0.14 |
| Allergic reaction | 8 (2) | 13 (3) | 0.38 |
| Mucositis | 171 (43) | 146 (37) | 0.08 |
| Infection without neutropenia | 181 (45) | 191 (48) | 0.52 |
| Febrile neutropenia | 355 (89) | 367 (92) | 0.18 |
| Peripheral neuropathy | 98 (25) | 102 (26) | 0.81 |
Data were represented as number (percentage). The Chi-square Independence test was used in statistical analysis. A p<0.05 was considered significant.
Significant zoledronate-emergent toxic effect.
As per CTCAE; v3.0. Paramedical staff (blinded regarding interventions) of the Cancer Hospital of China Medical University, China was involved in the evaluation of adverse effects.
Zoledronate increased the financial burden by 1445±12 ¥/for intravenous infusion.
Discussion
The addition of zoledronate to chemotherapy failed to improve the histopathological responses (p=0.12). These results are in line with the available OS2006 trial [3] but failed to reach the results of the experimental study [2] and a small report [7]. In humans, osteosarcoma has aggressiveness and biological heterogeneity [15]. In consideration of results of histological response, the addition of zoledronate to chemotherapy has no additive clinical benefits over chemotherapy alone in osteosarcoma patients.
The addition of zoledronate to chemotherapy improved event-free survival (p=0.04). These results of the study are in line with the OS2006 trial [3]. Zoledronate inhibits neoangiogenesis and attenuates osteosarcoma growth [2] and augmented the effect of a chemotherapy regimen [5]. The results of event-free survival suggest that the addition of zoledronate chemotherapy may improve results of osteosarcoma therapy.
Zoledronate with chemotherapy decreased overall survival (p=0.02). These results of the study are in line with the available trials [3,7], but male patients (p=0.74) and younger patients (25–40 years, p=0.06) had the same survival as that of patients receiving chemotherapy alone, with additional high event-free survival. These results of heterogeneity of age and sex were not in line with available trials [3,7]. A possible explanation for these results is that lack of statistically significance at the 5% level does not necessarily mean that zoledronate has no treatment-emergent adverse effect in young and male patients, simply that the association is unproven. Some results of the available studies are in line with the such results of the other trail, as zoledronate is more effective in younger patients than in older patients [16] and the risk of treatment is higher in Asian women and older men than in white patients [1]. However, addition of zoledronate to standard chemotherapy in patients with osteosarcoma is not recommended.
Zoledronate treatment has the risk of hypocalcemia, hypophosphatemia, fracture(s), flu-like syndrome, and ototoxicity. There were no orthopedic-related complications (except in female patients and patients age >40 years). These results of the study are in line with the available trials [3,7]. Zoledronate improves wound healing [17]. With respect to the results of a trial related to fracture and postoperative wound healing complications, there is a need to find an effective and safe alternative to zoledronate in female and older patients with osteosarcoma.
The OS2006 trial [3] and AOST06P1 study [7] have reported the same results for histological response, event-free survival, overall survival, and toxic effects, but disagree regarding sample size and evaluation methods. In the OS2006 trial enrollment was stopped prematurely [1] and enrolled white patients but included no Asian patients. Moreover, the AOST06P1 study [7] included only 24 patients, lacked a control group, and all enrolled patients were less than 40 years old at the time of enrollment.
We performed limb-sparing surgery after preoperative chemotherapies to remove the tumor. Limb-sparing surgeries and amputation surgeries have the same overall survival [18]. Amputee patients required more walking support than those who underwent limb-sparing surgery [19]. With respect to the method of surgeries performed in the trial, we were successful in improving the quality of life of patients, as well as improving with physical functioning.
We found more patients with lung metastases during 5-year follow-up for the standard chemotherapy with zoledronate group than with the chemotherapy alone group (9 vs. 1). The results of the trial were in line with the available tissue culture and cell line study [20] but not in line with the available cell line and animals study [21] and review article [6]. Zoledronate induces reduction of osteoclasts [22]. Osteoclasts inhibit the migration of osteosarcoma [20]. Therefore, there is a need for alternate chemotherapy treatment that reduces the risk of lung metastases.
There are several limitations of the study. Zoledronate also has extraskeletal effects [23]. The study did not evaluate the effects of zoledronate on the immune system and the other anti-tumor effects outside of bone. According to cell biology, zoledronate and methotrexate are antagonistic [5]. The surgeons’ experience in limb-sparing surgeries improved the outcomes [18], but our study did not discuss such parameters. Secondary amputation is an important parameter for site-specific control of functional outcomes for limb-sparing surgeries of osteosarcoma [18]. In our study, orthopedic surgeons did not perform secondary amputations in any patients. Analysis of quality of life was not carried out.
Conclusions
The addition of zoledronate to chemotherapy improved event-free survival of patients with osteosarcoma. However, zoledronate induced severe adverse effects and decreased overall survival of female patients and older patients. Zoledronate also increased the risk of pulmonary metastases. Therefore, addition of zoledronate to standard chemotherapy in high-grade resectable osteosarcoma is detrimental and should not be advised.
Acknowledgments
The authors are thankful for the medical and non-medical staff of the Cancer Hospital of China Medical University, China and the Graduate School, China Medical University, Shenyang, China.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Adler RA, El-Hajj Fuleihan G, Bauer DC, et al. Managing osteoporosis in patients on long-term bisphosphonate treatment: Report of a task force of the American society for bone and mineral research. J Bone Miner Res. 2016;31:16–35. doi: 10.1002/jbmr.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohba T, Cates JM, Cole HA, et al. Pleiotropic effects of bisphosphonates on osteosarcoma. Bone. 2014;63:110–20. doi: 10.1016/j.bone.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Piperno-Neumann S, Le Deley MC, Redini F, et al. Sarcoma Group of UNICANCER, French Society of Pediatric Oncology (SFCE), French Sarcoma Group (GSF-GETO) Zoledronate in combination with chemotherapy and surgery to treat osteosarcoma (OS2006): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2016;17:1070–80. doi: 10.1016/S1470-2045(16)30096-1. [DOI] [PubMed] [Google Scholar]
- 4.Meyers PA, Healey JH, Chou AJ, et al. Addition of pamidronate to chemotherapy for the treatment of osteosarcoma. Cancer. 2011;117:1736–44. doi: 10.1002/cncr.25744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horie N, Murata H, Kimura S, et al. Combined effects of a third-generation bisphosphonate, zoledronic acid with other anticancer agents against murine osteosarcoma. Br J Cancer. 2007;96:255–61. doi: 10.1038/sj.bjc.6603548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuasa T, Kimura S, Ashihara E, et al. Zoledronic acid – a multiplicity of anti-cancer action. Curr Med Chem. 2007;14:2126–35. doi: 10.2174/092986707781389600. [DOI] [PubMed] [Google Scholar]
- 7.Goldsby RE, Fan TM, Villaluna D, et al. Feasibility and dose discovery analysis of zoledronic acid with concurrent chemotherapy in the treatment of newly diagnosed metastatic osteosarcoma: A report from the Children’s Oncology Group. Eur J Cancer. 2013;49:2384–91. doi: 10.1016/j.ejca.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agha RA, Borrelli MR, Vella-Baldacchino M, et al. STROCSS Group. The STROCSS statement: Strengthening the Reporting of Cohort Studies in Surgery. Int J Surg. 2017;46:198–202. doi: 10.1016/j.ijsu.2017.08.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prescribing information for Zometa®. Beijing Novartis Pharma Co., Ltd; Beijing, China: http://chemocare.com/chemotherapy/drug-info/Zometa.aspx. [Google Scholar]
- 10.Kunisada T, Takeda K, Fujiwara T, et al. Limb salvage surgery for pelvic osteosarcoma. In: Ueda T, Kawai A, editors. Osteosarcoma. Springer; Tokyo: 2016. pp. 135–47. [Google Scholar]
- 11.Chawla S, Henshaw R, Seeger L, et al. Safety and efficacy of denosumab for adults and skeletally mature adolescents with giant cell tumour of bone: Interim analysis of an open-label, parallel-group, phase 2 study. Lancet Oncol. 2013;14:901–18. doi: 10.1016/S1470-2045(13)70277-8. [DOI] [PubMed] [Google Scholar]
- 12.Araki K, Ito Y, Takahashi S. Re: Superiority of denosumab to zoledronic acid for prevention of skeletal-related events: A combined analysis of three pivotal, randomised, phase 3 trials. Eur J Cancer. 2013;49:2264–65. doi: 10.1016/j.ejca.2013.02.036. [DOI] [PubMed] [Google Scholar]
- 13.Beutler E, Waalen J. The definition of anemia: What is the lower limit of normal of the blood hemoglobin concentration? Blood. 2006;107:1747–50. doi: 10.1182/blood-2005-07-3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albini A, Pennesi G, Donatelli F, et al. Cardiotoxicity of anticancer drugs: The need for cardio-oncology and cardio-oncological prevention. J Natl Cancer Inst. 2010;102:14–25. doi: 10.1093/jnci/djp440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hassan SE, Bekarev M, Kim MY, et al. Cell surface receptor expression patterns in osteosarcoma. Cancer. 2012;118:740–49. doi: 10.1002/cncr.26339. [DOI] [PubMed] [Google Scholar]
- 16.Eastell R, Black DM, Boonen S, et al. HORIZON Pivotal Fracture Trial. Effect of once-yearly zoledronic acid five milligrams on fracture risk and change in femoral neck bone mineral density. J Clin Endocrinol Metab. 2009;94:3215–25. doi: 10.1210/jc.2008-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matos MA, Tannuri U, Guarniero R. The effect of zoledronate during bone healing. J Orthop Traumatol. 2010;11:7–12. doi: 10.1007/s10195-010-0083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ayerza MA, Farfalli GL, Aponte-Tinao L, Muscolo DL. Does increased rate of limb-sparing surgery affect survival in osteosarcoma? Clin Orthop Relat Res. 2010;468:2854–59. doi: 10.1007/s11999-010-1423-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mei J, Zhu XZ, Wang ZY, Cai XS. Functional outcomes and quality of life in patients with osteosarcoma treated with amputation versus limb-salvage surgery: A systematic review and meta-analysis. Arch Orthop Trauma Surg. 2014;134:1507–16. doi: 10.1007/s00402-014-2086-5. [DOI] [PubMed] [Google Scholar]
- 20.Endo-Munoz L, Cumming A, Rickwood D, et al. Loss of osteoclasts contributes to development of osteosarcoma pulmonary metastases. Cancer Res. 2010;70:7063–72. doi: 10.1158/0008-5472.CAN-09-4291. [DOI] [PubMed] [Google Scholar]
- 21.Koto K, Horie N, Kimura S, et al. Clinically relevant dose of zoledronic acid inhibits spontaneous lung metastasis in a murine osteosarcoma model. Cancer Lett. 2009;274:271–78. doi: 10.1016/j.canlet.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 22.Labrinidis A, Hay S, Liapis V, et al. Zoledronic acid protects against osteosarcoma-induced bone destruction but lacks efficacy against pulmonary metastases in a syngeneic rat model. Int J Cancer. 2010;127:345–54. doi: 10.1002/ijc.25051. [DOI] [PubMed] [Google Scholar]
- 23.Junankar S, Shay G, Jurczyluk J, et al. Real-time intravital imaging establishes tumor-associated macrophages as the extraskeletal target of bisphosphonate action in cancer. Cancer Discov. 2015;5:35–42. doi: 10.1158/2159-8290.CD-14-0621. [DOI] [PMC free article] [PubMed] [Google Scholar]