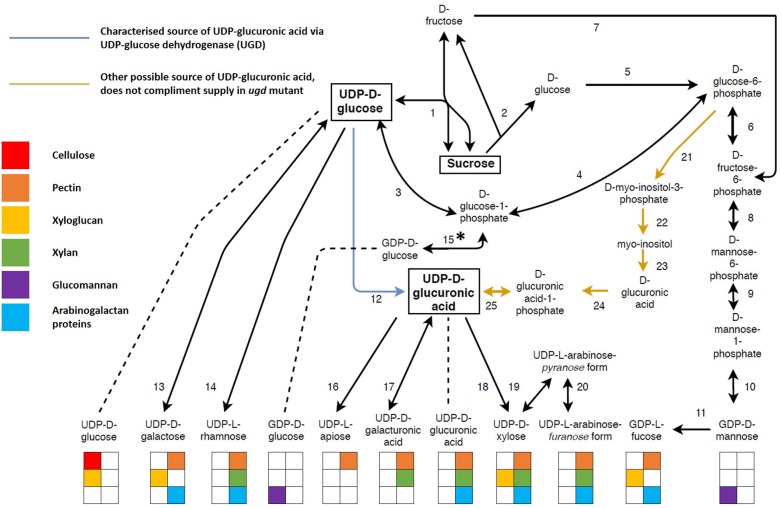
FIGURE 4.
The interconversion of sugar nucleotides derived from sucrose and their use in biopolymer biosynthesis. Sugar nucleotides are the precursors required for the biosynthesis of both primary and secondary cell wall biopolymers with most sugar nucleotides being used for more than one biopolymer. Sucrose is the main source of nucleotide sugars. Its reversible cleavage by sucrose synthase yield D-fructose and UDP-glucose. UDP-glucose serves as a precursor for many other nucleotide sugars, either as a direct source (UDP-galactose, UDP-rhamnose and UDP-glucuronic acid) or an indirect source via from UDP-glucuronic acid (UDP-xylose, UDP-galacturonic acid and UDP-apiose). UDP-glucuronic acid can either be derived from UDP-glucose (blue arrow) or glucose-6-phospate (orange arrows). The former pathway (12) is an almost exclusive source of UDP-glucuronic acid, whereas the latter pathway (21–25) is understudied and is unable to complement an ugd mutant. ∗The enzyme required for this reaction has not been identified in planta. The coloring of the blocks under each nucleotide sugar indicates the biopolymer for which the nucleotide sugar is a precursor. Unidirectional arrows indicate irreversible reactions whereas bidirectional arrows indicate reversible reactions. Key metabolites are highlighted in square boxes. The enzymes (1–25) represented by each number can be found in Supplementary Table S1, along with additional information such as gene ID and cellular localisation.