Summary
The aim of this study was to evaluate whether specific etiologies of neonatal seizures have distinct ictal electroclinical features. A systematic review of English articles using the PubMed database since 2004 (last update 9/26/16). Search terms included text words and Medical Subject Headings (MeSH) terms related to neonatal seizures. Eligible articles included reports of neonates with seizures with a full description of seizure semiology and electroclinical findings. Independent extraction of data was performed by 2 authors using predefined data fields, including study quality indicators. Data were collected for every individual patient described in the articles. The dataset was analyzed with the Fisher exact test. The initial search led to 8507 titles; using filters, 2910 titles and abstracts were identified, with 177 full texts selected to be read. Fifty‐seven studies were included in the analysis with 151 neonates (37.7 male and 62.9% term). Genetic etiologies (51%) and sequential seizures (41.1%) predominated in this sample and hypoxic‐ischemic encephalopathy (HIE) accounted for only 4%. The low prevalence of HIE observed was probably due to a publication bias. A significant association was found between etiology and seizure type: hemorrhage with autonomic seizures (P = 0.003), central nervous system (CNS) infection and stroke with clonic seizures (P = 0.042, P < 0.001, respectively), metabolic/vitamin‐related disorders, and inborn errors of metabolism with myoclonic seizures (P < 0.001). There were also specific electroencephalography (EEG) patterns seen with certain etiologies: vascular disorders and electrolyte imbalance with focal ictal discharges (P < 0.001, P = 0.049 respectively), vitamin‐related disorders with multifocal (P = 0.003), and all categories of genetic disorders with burst‐suppression (P < 0.001). Clonic and autonomic seizures were more frequently present with focal EEG abnormalities (P = 0.001 and P < 0.001), whereas tonic and myoclonic seizures present with burst‐suppression (P = 0.001, P = 0.005). In conclusion, our data suggest that specific associations of etiologies of neonatal seizures with distinct clinical features and EEG patterns might help in the decision to establish appropriate treatment.
Keywords: electroclinical features, neonatal EEG, neonatal seizures, semiology
1.
Key Points.
Specific etiologies of neonatal seizures may be associated with distinct clinical features and these associations might be useful in countries with limited resources
Specific electroclinical patterns may help in the recognition of the etiology of neonatal seizures
Widespread use of the Neonatal Task Force proposal should be helpful for collecting data in future studies
2. INTRODUCTION
Clinical identification of seizures in the neonate remains a challenge to neonatologists and other specialists caring for newborns. Neonates may demonstrate a vast repertoire of movements/behaviors seen in normal as well as sick newborns that may not be epileptic in origin.1, 2 Furthermore, the clinical features of seizures may be less clear compared to seizures in older children and adults. Although the latest World Health Organization (WHO) Guidelines on Neonatal Seizures strongly recommended that all clinical seizures should be confirmed by electroencephalography (EEG), often the equipment is not available in some settings or not at all times in many settings, and the decision to start treatment is based solely on clinical aspects.3 This can result either in misdiagnosis or overtreatment.1, 3, 4
Identifying associations between neonatal seizure etiology, semiology, and EEG features might help in the distinction of acute symptomatic seizures from seizures related to epilepsy, which influences the proper approach to treatment.
Previous studies that analyzed this relationship were not necessarily based on simultaneous EEG confirmation of the seizures5, 6, 7, 8, 9 or did not express, case by case, the electroclinical aspects of the ictal seizure.10, 11, 12, 13, 14
We conducted a systematic review in neonates with well‐documented electroclinical seizures to answer the following questions: (a) how etiology relates to semiology; (b) how etiology relates to EEG; and (c) how semiology relates to EEG features. We aimed to integrate the findings of existing studies to see if there is a relationship between ictal electroclinical features and etiology on seizures occurring during the neonatal period.
3. METHODOLOGY
For this systematic review, we used the PubMed database and search terms related to neonatal seizures (see below). The search period was from January 2004 to 2016 (last update 9/26/16), as before 2004, in the majority of studies, the seizure description was not confirmed by video‐EEG and was based on the clinical classification proposed by Volpe.15 The filters used were human studies and English language.
3.1. Inclusion criteria
Studies describing term and preterm neonates with seizures, with a description of the seizure semiology, concomitant EEG findings, and etiologic investigations.
Seizures from full‐term infants were included if they occurred within 30 days postdelivery.
Seizures from preterm infants were included if they occurred within the postmenstrual age (gestational age plus chronologic age in weeks) of 40 weeks.
3.2. Search strategy
The following search strategy was employed ((neonatal seizures) OR (neonatal convulsions) OR ((“Infant, Newborn”[MeSH]) AND seizures) OR ((“Infant, Newborn”[MeSH]) AND convulsions)).
3.3. Selection criteria
Studies were selected if the title and/or abstract suggested a description of semiology, EEG, or video‐EEG findings.
3.4. Exclusion criteria
Review articles, editorials, letters to the editor, articles without individual description of seizure semiology and/or EEG.
Articles that included EEG and semiology but were not within the neonatal period as described above.
3.5. Data collection and analysis
Titles and abstracts were first screened by 2 authors (MLN and RP) using predefined data fields. All full texts were read by the same authors, and the data were extracted and organized in an Excel table (Microsoft Corp.) and discussed within the group to assess quality indicators and reliability. The following variables were extracted: full bibliographic reference, number of patients, sex, gestational age, age at first seizure, etiology, and seizure semiology with EEG description. We used the list of etiologies as described in the 2017 International League Against Epilepsy (ILAE) classification of seizures and epilepsies16 but, because hypoxic‐ischemic encephalopathy (HIE) is so common in the neonatal period, we assigned it a special category. We have classified vascular etiologies and cortical malformations as a separate group, due to their frequency in this age group, instead of under the rubric of structural category as suggested in the ILAE classification. Thus, the etiologies were classified into the following 7 groups: (a) HIE, (b) cortical malformations, (c) central nervous system (CNS) infection, (d) metabolic (electrolyte imbalance, inborn errors of metabolism, vitamin‐related disorders, and withdrawal seizures), (e) genetic (channelopathies, chromosomal disorders, other gene disorders), (f) vascular (stroke and hemorrhage), and (g) Unknown. Although inborn errors of metabolism and vitamin‐related disorders can be included in either genetic or metabolic categories, we decided to include these cases in metabolic disorders. Similarly, although cortical malformations may have a genetic component, for the purpose of this report, we assigned them under the structural category. From the 57 articles included, we could evaluate electroclinical data from a total of 151 neonates (Table 1).
Table 1.
Full description of the sources
| GA/sex | Seizure onset (d) | Semiology (seizure description by author) | Seizure classification | Etiology | EEG |
|---|---|---|---|---|---|
| Pisano et al Epilepsia, 2015. N = 7/15 | |||||
| (NA)/female | 3rd | Tonic asymmetric | Tonic | KCNQ2 encephalopathy | Burst‐suppressiona |
| (NA)/female | 1st | Tonic asymmetric, apnea | Tonic | KCNQ2 encephalopathy | Burst‐suppressiona |
| (NA)/male | 3rd | Tonic asymmetric | Tonic | KCNQ2 encephalopathy | Focal (temporal) |
| (NA)/female | 1st | Clonic | Clonic | KCNQ2 encephalopathy | Multifocal |
| (NA)/male | 2nd | Tonic and clonic | Sequential (tonic, clonic) | KCNQ2 encephalopathy | Multifocal |
| (NA)/female | 3rd | Tonic asymmetric | Tonic | KCNQ2 encephalopathy | Multifocal |
| (NA)/male | 2nd | Tonic asymmetric | Tonic | KCNQ2 encephalopathy | Multifocal |
| Dereymaeker et al Eur J Pediatr Neurol, 2015. N = 1/1 | |||||
| Term/female | 9th | Clonic movements | Clonic | Transient hypothyroidism/viral encephalitis by HPeV type 3 | Multifocal |
| Cirillo et al Pediatrics, 2015. N = 2/2 | |||||
| Term/female | 5th | Myoclonic‐tonic and tonic seizures (rhythmic movements of extremities, eye deviation, oxygen desaturation). | Sequential (myoclonic, tonic, autonomic) | ALDH7A1 heterozygous mutation (c.328C.T; p.R1103) | Multifocal sharp waves |
| Term/female | 21st | Myoclonic jerks of arms and legs and tonic head deviation (tonic) | Sequential (myoclonic, tonic) | ALDH7A1—unknown | Bilateral continuous epileptiform discharges |
| Machado et al Einstein (São Paulo), 2015. N = 2/11 | |||||
| (NA)/(NA) | 9th | Multifocal clonic | Clonic | Left MCA ischemic stroke | Burst‐suppressiona |
| (NA)/(NA) | 1st | Focal clonic | Clonic | Left MCA ischemic stroke | Focal (left temporal) |
| Raimondi et al BMJ Case Report, 2015. N = 1/1 | |||||
| Preterm/female | 1st | Eyelid blinking, hypersalivation with orobuccal rhythmic movements | Sequential (automatisms, autonomic) | Pyridoxal 5‐phosphate deficiency, PNPO mutation | Burst suppression (background pattern) |
| Nascimento et al Pediatr Neurol, 2015. N = 1/1 | |||||
| Preterm/male | 20th | Crying, conjugate eye deviation to the right, myoclonus of the left eyelid, followed by chewing episodes with sialorrhea | Sequential (tonic, myoclonic, automatisms) | β‐oxidation defect from a D‐bifunctional protein deficiency | Multifocal |
| Fukasawa et al Am J Med Gent A, 2015. N = 2/7 | |||||
| Preterm/male | 28th | Apnea and tachycardia, sometimes followed by tonic posturing | Sequential (autonomic, tonic) | Trisomy 18 | Rhythmic spikes and slow waves of 2‐3 Hz from the right temporal‐occipital region |
| Term/female | 2nd | Apnea | Autonomic | Trisomy 18 | Rhythmic spikes and slow waves of 1‐2 Hz from the right temporal‐rolandic‐occipital region |
| Guerin et al J Child Neurol, 2015. N = 1/1 | |||||
| Preterm/female | 1st | Fragmentary and generalized myoclonic jerks | Myoclonic | Pyridox(am)ine‐5‐phosphate oxidase deficiency | Burst‐suppression (background pattern) |
| Spagnoli et al J Child Neurol, 2015. N = 2/2 | |||||
| Preterm/male | 5th | Multifocal clonic | Clonic | IVH grade III with posthemorrhagic hydrocephalus | Multifocal discharges, alpha‐beta range, left centrotemporal or posterior emphasis, less frequently with a right temporal onset |
| Preterm/female | 30th | Clonic events | Clonic | IVH with posthemorrhagic hydrocephalus | Low‐voltage alpha‐beta activity over the anterior regions, mainly expressed over the right |
| Paddock et al J Neonatal Perinatal Med, 2014. N = 1/1 | |||||
| Term/female | 1st | Clonic (right hand and leg) | Clonic | Left MCA ischemic stroke | Focal spikes left hemisphere (aEEG) |
| Saitsu et al J Hum Genet, 2014. N = 2/2 (siblings) | |||||
| Term/female | 7th | Focal clonic followed by generalized tonic‐clonic | Sequential (clonic, tonic) | Ohtahara (BRAT1 mutation) | Burst‐suppression (background pattern) |
| Term/female | 1st | Generalized myoclonic seizures and partial clonic, after tonic and apnea | Sequential (myoclonic, clonic, tonic, autonomic) | Ohtahara | Burst‐suppression (background pattern) |
| Ito et al J Perinatol, 2014. N = 1/1 | |||||
| Term/female | 1st | Deviation of eyeballs, nystagmus, twitching of the eyelids, tonic or clonic activities of the limbs or apnea | Sequential (tonic, autonomic) | Holoprosencephaly | Low‐voltage fast rhythms followed by slow waves of increasing amplitude C3‐C4 (aEEG) |
| Allen et al Epilepsia, 2014. N = 3/3 | |||||
| Term/female | 4th | Mainly clonic, but also tonic, minor cyanosis | Sequential (clonic, tonic, autonomic) | BFNS‐KCNQ2c.419_430dup | Bilateral independent high‐amplitude sharp waves of 1 Hz, normal background |
| Term/female | 6th | Clonic | Clonic |
BFNS‐KCNQ3 c.989G>A |
Excessive sharp waves, normal background |
| Term/male | 1st | Tonic arm and trunk with cyanosis, grunting and duskiness followed by apnea and hypoxia | Sequential (tonic, autonomic) | KCNQ2‐.881C>T encephalopathy | Ictal pattern: focal recruiting rhythm right parietal region Interictal: multifocal discharges, followed by background attenuation |
| Low et al PLoS ONE, 2014. N = 9/9 | |||||
| Term/male | 1st | Clonic right arm | Clonic | Left MCA ischemic stroke | Focal spikes, left central |
| Term/male | 2nd | Dusky episodes | Autonomic | Left MCA ischemic stroke | Focal spikes. left central |
| Term/male | 1st | Clonic left side | Clonic | Right MCA ischemic stroke | Focal spikes, right central |
| Term/female | 1st | Clonic right side | Clonic | Right/left MCA ischemic stroke | Focal spikes, polyspikes left central |
| Term/female | 2nd | Clonic left leg | Clonic | Right MCA ischemic stroke | Focal spikes, polyspikes right central |
| Term/female | 2nd | Clonic right side | Clonic | Left MCA ischemic stroke | Focal spikes, left central |
| Term/male | 1st | Clonic right arm | Clonic | Left MCA ischemic stroke | Focal spikes, left central |
| Term/male | 1st | Clonic right arm | Clonic | Right/left MCA ischemic stroke | Focal spikes, polyspikes left central |
| Term/male | 2nd | Clonic left leg | Clonic | Right MCA ischemic stroke | Focal spikes, polyspikes right central |
| Pariani et al Pediatr Infect Dis J, 2014. N = 1/2 | |||||
| Term/female | 9th | Myoclonic seizures, apnea and staring | Sequential (myoclonic, autonomic, behavioral arrest) | Parechovirus 3 encephalitis | Paroxysmal activity in the left and right hemisphere |
| Zerem et al Eur J Paediatr Neurol, 2014. N = 2/2 | |||||
| Term/male | 1st | General tonic extension, cry and usually desaturation | Sequential (tonic, autonomic) | SCN2A mutation (Ohtahara) | Burst‐suppression (background pattern) |
| Term/male | 1st | Tonic seizure, eye deviation, bradycardia | Sequential (tonic, autonomic) | SCN2A mutation (Ohtahara) | Ictal: focal discharges right frontal region Interictal: Burst‐suppression background |
| Ansary et al Singapore Med J, 2014. N = 1/1 | |||||
| Preterm/female | 2nd | Multifocal myoclonic (both arms and legs) | Myoclonic | Venlafaxine withdrawal | Focal sharp waves (aEEG) |
| Kharoshankaya et al Dev Med Child Neurol, 2014. N = 1/1 | |||||
| Term/male | 1st | Clonic (right arm and leg) associated with mouthing and cyanosis | Sequential (clonic, automatisms) | Thalamic infarction | Low voltage (<10 μv) focal left‐sided biphasic spike‐wave discharges |
| Fong et al Pediatr Infect Dis J, 2014. N = 1/1 | |||||
| Term/female | 13th | Focal clonic arm | Clonic | Herpes simplex virus type 1 | Focal epileptiform discharges over the midline‐vertex and right frontal‐midline regions |
| Numis et al Neurology, 2014, N = 3/3 | |||||
| Preterm/NA | 4th day | Tonic head, conjugate eye, and mouth deviation, unilateral tonic abduction of the limbs, apnea, and desaturation | Sequential (tonic, autonomic) | KCNQ2 epileptic encephalopathy | Low‐voltage fast activity followed by recruiting spikes or theta rhythms arising mainly from the central regions of either hemisphere, followed by focal spike‐wave complexes and prolonged focal or diffuse postictal suppression |
| Term/NA | 1st day | Tonic head, conjugate eye, and mouth deviation, unilateral tonic abduction of the limbs, apnea, and desaturation | Sequential (tonic, autonomic) | KCNQ2 epileptic encephalopathy | Focal low‐voltage fast activity followed by rhythmic theta rhythm from the fronto central region of both hemispheres, alternatively followed by diffuse marked postictal suppression lasting up to 8 minutes |
| Term/NA | 1st day | Tonic head, conjugate eye, and mouth deviation, unilateral tonic abduction of the limbs, apnea, and desaturation | Sequential (tonic, autonomic) | KCNQ2 epileptic encephalopathy | Low‐voltage fast activity followed by focal theta rhythms involving the right or left hemisphere |
| Porri et al Neuropediatrics, 2014. N = 1/1 | |||||
| Preterm/male | 1st | Erratic myoclonic jerks involving all four extremities | Myoclonic | Pyridoxal‐5′‐Phosphate Oxidase Deficiency | Burst‐suppression (ictal) |
| Khajeh et al J Child Neurol, 2014. N = 1/1 | |||||
| Term/female | 1st | Apnea | Autonomic | Polymicrogyria left temporal and frontal lobes | Left temporal 9‐10 Hz activity, evolving into 2‐ to 3‐Hz sharp and slow‐wave activity |
| Weckhuysen et al Neurology, 2013. N = 11/11 | |||||
| (NA)/female | 1st | Tonic asymmetrical with apnea, bradycardia and desaturation, continuous complex movements of legs | Sequential (tonic, autonomic, clonic or automatisms) | KCNQ2 mutation | Burst‐suppressiona |
| (NA)/female | 2nd | Apnea, erratic myoclonic and tonic contraction | Sequential (autonomic, myoclonic, tonic) | KCNQ2 mutation | Burst‐suppressiona |
| (NA)/male | 2nd | Tonic generalized | Tonic | KCNQ2 mutation | Burst‐suppressiona |
| (NA)/male | 1st | Tonic generalized with apnea, grimacing, followed by mastication and sialorrhea | Sequential (tonic, autonomic) | KCNQ2 mutation | Burst‐suppressiona |
| (NA)/male | 2nd | Tonic with pursing of lips, clenching of eyes and cyanosis, sometimes eye deviation and flickering of eyeballs | Sequential (tonic, automatism, autonomic) | KCNQ2 mutation | Burst‐suppressiona |
| (NA)/female | 2nd | Tonic asymmetrical with sucking movements of mouth | Sequential (tonic, automatism) | KCNQ2 mutation | Burst‐suppressiona |
| (NA)/female | 1st | Tonic asymmetrical with apnea | Tonic | KCNQ2 mutation | Multifocal |
| (NA)/male | 1st | Tonic asymmetrical with apnea | Tonic | KCNQ2 mutation | Focal evolving to multifocal |
| (NA)/female | 3rd | Tonic asymmetrical followed by hemiclonic | Sequential (tonic, clonic) | KCNQ2 mutation | Multifocal |
| (NA)/female | 1st | Tonic generalized | Tonic | KCNQ2 mutation | Focal spike waves |
| (NA)/female | 2nd | Tonic asymmetrical and apnea | Tonic | KCNQ2 mutation | Bilateral spikes |
| Borkenhagen et al Pediatr Neurol, 2013. N = 1/1 OK | |||||
| Term/female | 5th | Clonic right foot, with subsequent multifocal clonic (arms and legs independently) | Clonic | Hypocalcemia | High‐voltage, rhythmic spike‐wave discharges, left vertex region with spread into the left posterior temporal, left parietal, and right parietal regions. |
| Serino et al Epileptic Disord, 2013. N = 1/1 | |||||
| Term/male | 3rd day | Focal, tonic seizures with head deviation, asynchronous and asymmetrical clonic jerks, eyelid myoclonias, and polypnea | Sequential (tonic, clonic) | KCNQ2 epileptic encephalopathy | Focal, low‐voltage, fast activity, followed by recruiting theta rhythms and bilateral, focal, spike‐wave complexes, alternatively localized to one hemisphere and subsequently diffusing to the other |
| Mihl et al Orphanet J Rare Dis, 2013. N = 16/16 | |||||
| Term/(NA) | 1st | Clonic and tonic | Sequential (clonic, tonic) | KCNQ2 mutations | Burst‐suppressiona |
| Preterm/(NA) | 15th | Myoclonic | Myoclonic | KCNQ2 mutations | Periods of flatnessa |
| Term/(NA) | 3rd | Tonic, pallor 2 | Tonic | KCNQ2 mutations | Burst‐suppressiona |
| Term/(NA) | 2nd | Tonic and hypotonic. Epileptic spasms | Sequential (tonic, epileptic spasms) | KCNQ2 mutations | Burst‐suppressiona |
| Term/(NA) | 2nd | Tonic and tonic‐clonic, cyanosis | Sequential (tonic, clonic, autonomic) | KCNQ2 mutations | Generalized spikes predominating on the left hemisphere followed by suppression burst. a |
| Term/(NA) | 2nd | Left and right clonic jerks, facial cyanosis. | Clonic | KCNQ2 mutations | Burst‐suppressiona |
| Term/(NA) | 1st | Isolated cyanosis, than recurrent hypertonic posture | Sequential (autonomic, tonic) | KCNQ2 mutations | Burst‐suppressiona |
| (NA)/(NA) | 1st | Tonic asymmetric. | Tonic | KCNQ2 mutations | Bursts of multifocal spikes . |
| Term/(NA) | 3rd | Tonic | Tonic | KCNQ2 mutations | Burst‐suppressiona |
| Term/(NA) | 1st | Tonic and/or clonic | Sequential (tonic, clonic) | KCNQ2 mutations | Burst of asynchronous spikes and sharp waves. Periods of discontinuity with flatness no typical burst suppression |
| Term/(NA) | 1st | Tonic and cyanosis | Tonic | KCNQ2 mutations | Left or right spikes on a moderately abnormal background |
| Term/(NA) | 4th | Asymmetric tonic extension of one limb. Bilateral clonic seizures. Apnea. | Sequential (tonic, clonic, autonomic) | KCNQ2 mutations | Burst‐suppressiona |
| Term/(NA) | 4th | Clonic hemi corporeal, left or right | Clonic | KCNQ2 mutations | Prolonged periods of flatness. Discontinuous.a |
| Term/(NA) | 1st | Tonic | Tonic | KCNQ2 mutations | Multifocal slow waves, left frontal and right occipital spikes. Asymmetrical suppression‐burst |
| Preterm/(NA) | 8th | Myoclonic | Myoclonic | KCNQ2 mutations | Burst‐suppressiona |
| Term/(NA) | 2nd | Bilateral tonic clonic and right clonic | Sequential (tonic, clonic) | KCNQ2 mutations | Slow waves with asynchronous bilateral spikes and intermittent flattening |
| Tanriverdi et al Brain Dev, 2013. N = 1/1 | |||||
| Term/female | 20th day | Focal seizures followed by generalization | Sequential (no specific description) | Sturge‐Weber | Isolated sharp spike‐wave discharges at parietal right hemisphere and at the frontotemporal areas of left hemisphere |
| Kato et al Epilepsia, 2013. N = 12/12 | |||||
| (NA)/female | 1st | Tonic, eye deviation | Tonic | KCNQ2 mutation | Burst‐suppression, asymmetric a |
| (NA)/male | 3rd | Tonic | Tonic | KCNQ2 mutation | Multifocal sharp waves |
| (NA)/male | 5th | Left sided tonic | Tonic | KCNQ2 mutation | Burst‐suppression, brief suppression a |
| (NA)female | 2nd | Tonic | Tonic | KCNQ2 mutation | Burst‐suppression, asymmetric a |
| (NA)/male | 1st | Tonic | Tonic | KCNQ2 mutation | Burst‐suppression, brief suppression a |
| (NA)/male | 30th | Asymmetric tonic | Tonic | KCNQ2 mutation | Burst‐suppressiona |
| (NA)/male | 14th | Tonic | Tonic | KCNQ2 mutation | Burst‐suppression, asymmetric a |
| (NA)/male | 2nd | Tonic | Tonic | KCNQ2 mutation | Burst‐suppression, brief suppression a |
| (NA)/female | 2nd | Tonic | Tonic | KCNQ2 mutation | Burst‐suppression, like hypsarrhythmia a |
| (NA)/female | 14th | Generalized tonic | Tonic | KCNQ2 mutation | Burst‐suppression, brief suppression a |
| (NA)/male | 1st | Postural tonic | Tonic | KCNQ2 mutation | Burst‐suppressiona |
| (NA)/female | 3rd | Tonic, facial clonic | Sequential (tonic, clonic) | KCNQ2 mutation | Burst‐suppression, asymmetric a |
| Simoneti et al Epilepsia, 2012. N = 2/2 | |||||
| Term/female | 1st | Unusual cry, wide opening of the eyes, flushing, and bulbar and head deviation to the right | Sequential (autonomic, tonic) | Duplication of the sodium channel gene cluster on 2q24 5.1 | Right centrotemporal, also bicentral, slow, repetitive spike wave activity, followed by background slowing. |
| Term/female | 3rd | Focal tonic, multifocal clonic seizures, starts with central cyanosis and head deviation | Sequential (autonomic, tonic, clonic) | Duplication of the sodium channel gene cluster on 2q24 | Generalized suppression of the background activity, followed by sharp and slow waves, secondarily generalizing. |
| Riesgo et al Neuropediatrics, 2012. N = 3/3 | |||||
| Preterm/male | 10th | Apnea and desaturation | Autonomic | Undetermined | Focal rhythmic activity on the left temporal region. |
| Preterm/female | 22nd | Apnea | Autonomic | Periventricular leukomalacia | Multifocal paroxysms occurred mainly in the right temporal region |
| Preterm/female | 2nd | Apnea, clonic upper limbs | Sequential (autonomic, clonic) | Undetermined 7.0 | Multifocal paroxysms and EEG seizures in both hemispheres mainly at left temporal region |
| Cusmai et al Eur J Pediatr Neurol, 2012. N = 3/3 | |||||
| Term/female | 2nd | Myoclonic seizures and epileptic tonic spasms. | Myoclonic | Nonketotic hyperglycinemia | Burst‐suppression (background pattern) |
| Term/male | 2nd | Myoclonic jerks and infantile spasms | Myoclonic | Nonketotic hyperglycinemia | Burst‐suppression (background pattern) |
| Term/male | 1st | Myoclonic jerks and tonic spasms | Myoclonic | Nonketotic hyperglycinemia | Burst‐suppression (background pattern) |
| Vatta et al J Child Neurol, 2012. N = 1/1 | |||||
| Term/male | 14th day | Opening of the eyes followed by body stiffening and breathing difficulties, clonic right arm | Sequential (tonic, autonomic, clonic) | STXBP1 mutation 5.3 | Focal discharges, left central region, alpha/theta range |
| Weckhuysen et al Ann Neurol, 2012. N = 6/8 | |||||
| (NA)/female | 2nd day | Apnea, generalized stiffening with facial suffusion, followed by pallor and cyanosis | Sequential (autonomic, tonic) | KCNQ2 epileptic encephalopathy | Continuous multifocal and bilaterally synchronous epileptiform activity. |
| (NA)/female | 3rd day | Stiffening, head and eye deviation and tonic posturing | Sequential (autonomic, tonic) | KCNQ2 epileptic encephalopathy | Centroparietal ictal rhythm evolving to high‐voltage slowing (right‐sided in 2 seizures and left‐sided in 1) |
| (NA)/male | 2nd day | Generalized tonic with clonic components, lip smacking, back arching, apnea | Sequential (tonic, clonic, automatism, autonomic) | KCNQ2 epileptic encephalopathy | Multifocal epileptic activity most frequently seen in left temporal and right frontal regions. |
| (NA)/female | 3rd day | Tonic seizure, followed by myoclonic jerks and nystagmus | Sequential (tonic, myoclonic) | KCNQ2 epileptic encephalopathy | Burst‐suppressiona |
| (NA)/male | 3rd day | Tonic extension with clonic movements left hemicorpus and eyelid myoclonia | Sequential (tonic, myoclonic) | KCNQ2 epileptic encephalopathy | Burst‐suppressiona |
| (NA)/female | 2nd day | Tonic extension, high pitch cry, cyanosis and bradypnea, eventually with myoclonias (arms) | Sequential (tonic, myoclonic, autonomic) | KCNQ2 epileptic encephalopathy | Burst‐suppressiona |
| Blumkin et al Eur J Pediatr Neurol, 2012. N = 1/1 | |||||
| Term/male | 2nd | Multifocal clonic | Clonic | KCNQ2 mutation | Generalized spike and wave (2‐2.5 Hz) with phase reversal in the rolandic area bilaterally. |
| Castro‐ Conde et al Pediatrics, 2012. N = 2/2 | |||||
| Term/male | 1st day | Eye opening, tachycardia, tonic eye deviation to the left, slow blinking, mouth movements, right arm abduction with clenched fist and eye deviation to the right followed by apnea | Sequential (autonomic, automatisms, tonic) | Ischemic stroke | Rhythmic sharp waves left temporal followed by generalized background suppression |
| Term/female | 2nd day | Apnea | Autonomic | Unknown | Focal occipital discharges |
| Hirata et al Neuropediatrics, 2011. N = 1/1 | |||||
| Term/female | 16th | Clonic seizures right arm and leg | Clonic | Coxsackie B2 Meningoencephalitis | Multifocal spikes |
| Milh et al Epilepsia, 2011. N = 4/5 | |||||
| (NA)/(NA) | 1st | Clonic asynchronous | Clonic | STXBP1 (MUNC18‐1) mutations | Burst‐suppressiona |
| (NA)/(NA) | 1st | Clonic asynchronous | Clonic | STXBP1 (MUNC18‐1) mutations | Burst‐suppressiona |
| (NA)/(NA) | 3rd | Epileptic spasms | Epileptic spasms | STXBP1 (MUNC18‐1) mutations | Burst‐suppressiona |
| (NA)/(NA) | 1st | Epileptic spasms | Epileptic spasms | STXBP1 (MUNC18‐1) mutations | Burst‐suppressiona |
| Walsh et al Dev Med Child Neurol, 2011. N = 1/1 | |||||
| Term/female | 1st to 2nd day | Lip smacking and tonic‐clonic | Sequential (automatisms, tonic, clonic) | Ischemic stroke | Bursts sharp waves left hemisphere |
| Millet et al Eur J Pediatr Neurol, 2011. N = 1/1 | |||||
| Term/male | 1st | Clonic | Clonic | Pyridoxine‐dependent epilepsy with mutation in the ALDH7A1 gene | Rhythmic spikes that predominated in the right or left hemisphere, in the temporal region. Burst‐suppression background |
| Heron et al Epilepsia, 2010. N = 1/1 ok | |||||
| Term/male | 4th day | Myoclonic | Myoclonic | QT prolongation mutation in SCN5A c.4868G>A (p.R1623Q) | Bilateral rhythmic epileptic discharges predominantly posterior (O1 and O2) with a right‐sided emphasis. |
| Gibson & Bharti. Tenn Med, 2010. N = 2/2 ok | |||||
| Term/female | 1st | Focal clonic left leg | Clonic | Left MCA ischemic stroke | Rhythmic discharges left temporal spreading to frontal/central regions |
| Term/female | 1st | Focal tonic clonic, smacking lips, tongue deviation | Sequential (tonic, clonic, automatisms) | Right MCA ischemic stroke | Multifocal sharp waves |
| Schmitt et al Dev Med Child Neurol, 2010. N = 1/5 ok | |||||
| Term/female | 7th | Focal clonic | Clonic | Pyridoxine‐dependent epilepsy | Central spikes |
| Okumura et al Brain Dev, 2008. N = 3/9 ok | |||||
| Preterm/male | 25th day | Autonomic 7 | Autonomic | Severe hypotension hyperkalemia | Right temporal rhythmic slow voltage spikes |
| Preterm/female | 1st day | Apnea | Autonomic | Neonatal encephalopathy | Rhythmic spikes, right temporal |
| Preterm/female | 1st day | Apnea | Autonomic | Subarachnoid hemorrhage | Rhythmic spikes, left temporal |
| Nunes et al Arq Neuropsiquiatr (São Paulo), 2008. N = 6/101 | |||||
| Term/female | 4th day | Clonic left arm, after left leg, chewing movements | Sequential (clonic, automatisms) | Benign familial neonatal seizures | Rhythmic discharges theta range right central and temporal with propagation to left central |
| Term/male | 1st day | Multifocal clonic | Clonic | Hypoxic‐ischemic encephalopathy | Rhythmic spikes, right temporal and rolandic |
| Term/male | 2nd day | Clonic left arm and face | Clonic | Abstinence | Rhythmic discharges, right occipital |
| Term/female | 2nd day | Clonic focal left arm | Clonic | Right MCA ischemic infarct | Rhythmic spike and slow wave right rolandic, with propagation to right frontal |
| Term/female | 2nd day | Apnea | Autonomic | Hypoxic‐ischemic encephalopathy | Rhythmic discharges, left occipital |
| Term/female | 1st day | Clonic focal right arm, blinking right eye | Clonic | Left MCA ischemic infarct | Rhythmic discharges, delta range, left rolandic |
| Kubota et al Brain Dev, 2008. N = 1/1ok | |||||
| Term/female | 2nd day | Clonic left side, with open eyes deviating to the left, and automatism around the mouth | Sequential (clonic, tonic, automatism) | Hypoxic‐ischemic encephalopathy | Semi‐rhythmic, repetitive spikes predominantly in the right central region |
| Shah et al N Engl J Med, 2008. N = 1/1 | |||||
| Term/female | 1st | Apnea | Autonomic | Left MCA ischemic stroke | Sharp waves left temporal |
| Vecchi et al Epileptic Dis, 2007. N = 1/1 | |||||
| Preterm/male | 7th | Behavioral arrest, staring, apnea, deviation of the head and the eyes to the right, dystonic posture of the left hand and bilateral, automatic hand movements | Sequential (behavior arrest, autonomic, tonic) | Undetermined | Rapid rhythms of low voltage in the right temporal region followed by theta rhythmic activity and rhythmical sharp and wave complexes |
| Gorman & Soul. Pediatr Neurol, 2007. N = 1/1 | |||||
| Term/male | 3 rd | Tonic‐clonic | Sequential (tonic, clonic) | Hypocalcemia | Left central and vertex sharp waves that spread to right side |
| Sirsi et al Pediatr Neurol, 2007.N = 3/3 | |||||
| Term/male | 1st | Apnea, conjugate eye deviation to the right, focal clonic (right‐arm) | Sequential (autonomic, tonic, clonic) | Hemorrhage (left temporal lobe) | Left temporal sharp rhythmic delta activity, evolving into alpha with admixed theta sharp and slow‐wave |
| Term/male | 1st | Apnea | Autonomic | Intraparenchymal hemorrhage (right temporal) and subdural (right tentorium) hematoma | Focal activity (right hemisphere) |
| Term/male | 1st day | Apnea | Autonomic | Right temporal hemorrhagic infarct | Right temporal rhythmic spike and wave activity |
| Lin et al Arq Neuropsiquiatr (São Paulo), 2007. N = 1/1 | |||||
| Term/female | 1st | Focal myoclonic left arm and leg) tonic eye and head deviation to the right, eyelid blinking and oromandibular movements | Sequential (myoclonic, tonic, autonomic) | Pyridoxine‐dependent epilepsy | High‐voltage spike and polyspike‐wave complexes lateralized to the right cerebral hemisphere |
| Hmaimess et al Pediatr Neurol, 2007. N = 1/1 | |||||
| Term/male | 1st | Lateral deviation of the head and eyes, fixed sight, clonic jerks on one side of the body followed by clonic jerks of the other side | Sequential (tonic, clonic) | Malignant migrating partial seizures (etiology unknown) | Low‐voltage fast right central and occipital activity (9‐10 Hz), anterior ipsilateral flattening, followed by increased amplitude and slowing to theta and delta rhythmic activity left side |
| Spinosa et al Arq Neuropsiquiatr (São Paulo), 2006. N = 1/1 | |||||
| Term/male | 1st | Focal clonic (right hemiface and arm) | Clonic | X‐linked lissencephaly with ambiguous genitalia (XLAG) | Right midtemporal, central and occipital discharges |
| Cherian et al Clin EEG Neurosci, 2006. N = 1/1 | |||||
| Term/(NA) | (NA) | Nystagmoid movements | Automatism | Hypoxic‐ischemic encephalopathy | Focal bilateral occipital discharges |
| Schmitt et al Epileptic Res, 2005. N = 6/6 | |||||
| Term/(NA) | 1st day | Tonic followed by asymmetric clonic | Sequential (tonic, clonic) | Pyridoxine‐ dependent seizures | Bilateral synchronous spike‐wave discharges followed by suppression |
| Term/(NA) | 1st day | Multifocal myoclonic jerks, intermittent tonic posturing or spasms, eye deviations and abnormal oral and mimic movements | Sequential (myoclonic, tonic, epileptic spasms, automatisms) | Pyridoxine‐ dependent seizures | Voltage suppression in EEG followed by bilateral synchronous spike‐wave discharges |
| Term/female | 1st day | Tonic clonic‐myoclonic seizures | Sequential (tonic, clonic, myoclonic) | Undetermined | Rhythmic and sharp activity alternated from both hemispheres |
| Term/male | 1st day | Tonic‐clonic | Sequential (tonic, clonic) | KCNQ2 | Voltage suppression, bilateral rhythmic alpha discharges |
| Term/female | 1st day | Slow dystonic movements followed by focal clonic on right arm and leg | Sequential (tonic, clonic) | Undetermined | Voltage suppression rhythmic and sharp left hemisphere |
| Term/male | 8th day | Tonic clonic | Sequential (tonic, clonic) | Undetermined | Multifocal |
| Al‐Futaisi et al Clin Neurophysiol, 2005. N = 1/1 | |||||
| Term/female | 5 days | Tonic spasms | Epileptic spasms | EIEE (etiology unknown) | Burst‐suppression (ictal) |
| Schulzke et al J Perinatal Med, 2005. N = 6/9 | |||||
| Preterm/male | 1st | Focal clonic | Clonic | Left MCA ischemic stroke | Focal left discharges |
| Term/male | 1st | Apnea plus tonic and clonic | Sequential (autonomic, tonic, clonic) | Hemorrhage (left parietooccipital region) | Focal left discharges |
| Term/female | 1st | Apnea plus tonic and clonic | Sequential (autonomic, tonic, clonic) | Left MCA ischemic stroke | PLEDS left |
| Term/female | 2nd | Focal clonic | Clonic | Left MCA ischemic stroke | Focal left discharges |
| Term/male | 3rd | Focal clonic | Clonic | Left MCA ischemic stroke | Focal left discharges and sharp/slow waves right |
| Term/female | 2nd | Focal clonic | Clonic | Left MCA ischemic stroke | Focal left discharges |
| Tramonte & Goodkin. J Perinatol, 2004. N = 1/1 | |||||
| Term/male | 1st | Apnea | Autonomic | Intraparenchymal hemorrhage (right temporal) | Sharply contoured alpha activity evolving into periodic sharp and slow wave activity followed by rhythmic delta activity, right centrotemporal |
AED, antiepileptic drug; EIEE, early infantile epileptic encephalopathy; GA, gestational age, seizure onset expressed in days of life; IVH, intraventricular hemorrhage; LMCA, left middle cerebral artery; N, number of patients included/number of patients available in the study; PLEDs, pseudoperiodic epileptiform discharges; RMCA, right middle cerebral artery; (NA), not available. In this table we have maintained the description of seizure semiology and EEG findings as it is cited in the original article.
Indicates when burst‐suppression was not clearly defined as ictal or interictal pattern/background abnormality.
Semiology was described as clonic, tonic, myoclonic, automatisms, epileptic spasms, and autonomic, when it was possible to identify the main clinical feature of the seizure. We used the term sequential seizures, according to the report of ILAE Neonatal Task Force (available online)17 and the 2017 ILAE classification manual, in situations when it was difficult to identify the dominant feature, typically in longer seizures where a sequence of clinical features was seen, often with changing lateralization.18 The articles were reviewed by 3 of the authors (RP, MN, and EY) for agreement in seizure type based on the seizure semiology described in the articles. The ictal EEG patterns were classified as focal (unilateral or bilateral onset), multifocal (more than 2 different and independent foci), or burst‐suppression. However, in many articles, burst‐suppression was described as a background pattern, and the authors did not specify if the seizure episode correlated with the diffuse suppression. In Table 1, we documented how the various authors used the term burst‐suppression in their papers.
The guidelines from preferred reporting items for systematic review and meta‐analysis and a measurement tool to assess systematic reviews methodology were used to analyze papers included in the study.19, 20, 21 We initially planned to use meta‐analytic techniques.22 However, because there was a large number of studies with only single cases, confidence intervals could not be calculated, thereby preventing the meta‐analysis calculations. Nevertheless, we collected and included data for every individual patient described in the studies. Furthermore, the dataset was analyzed as if it came from a single study, with the Fisher exact test. Significant results were considered when the P‐value was <0.05. When the P‐value was equal to 0.000, we have expressed it as P < 0.001.
The data were analyzed looking at combinations of clinical semiology of the seizures, etiology, and EEG patterns. In the statistical analysis, if the initial evaluation of the data suggested an association between or among the groups of etiology, all the other categories were grouped together. For example, if an association between a channelopathy (genetic group) and tonic seizures was observed, the statistical analysis (Fisher exact test) was done by grouping all other etiologies (ie, channelopathy vs all others) and all other seizure types (ie, tonic vs all others) and creating a 2 × 2 table. The same approach was applied to compare seizure type and EEG as well as etiology and EEG.
4. RESULTS
Figure 1 demonstrates the steps of the systematic review and inclusion of the articles. From the initial search, there were 8507 titles. After applying filters (period 2004‐2016, human studies, English language), the number decreased to 2910. After reviewing the titles and abstracts, we excluded review articles, editorial letters, and expert opinions. This left 177 full‐text articles. Of these, 117 articles were excluded for the following reasons: there was no individual semiology description of the seizures; seizures did not occur during the neonatal period, or there were only descriptions of interictal EEG or no neonatal EEG description at all; and finally, there was no clinical‐EEG correlation. Three articles were not available online. We therefore identified 57 articles that provided data to correlate the EEG patterns with clinical semiology of seizures and the description of the etiologic diagnosis (Table 1).
Figure 1.
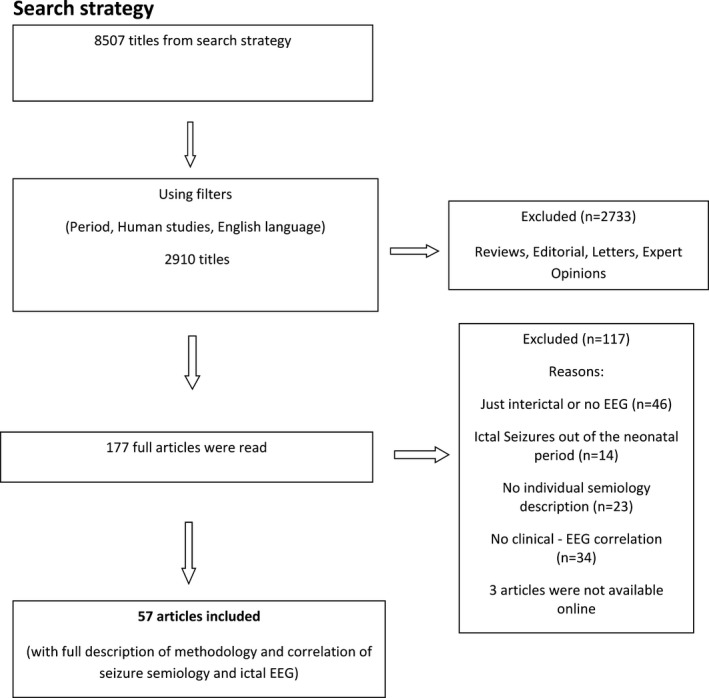
Search strategy
Although these studies included data on 282 neonates, several cases were duplicates or information was not available to compare individual semiology vs etiology vs EEG. Thus, 151 neonates were included in the final analysis. In each study, the number of patients included varied from 1 to 16 (median 1, mean ± standard deviation [SD] 2.78 ± 3.20).
Of the neonates included, 37.7% were male and 45.0% female; 62.9% were born at term. Information regarding sex or gestational age was not available in 17.2% and 24.5% of the cases, respectively. Table 2 summarizes the semiology, etiology, and EEG findings.
Table 2.
General characteristics of the 151 included neonates
| Sexa (n = 125) |
Male 37.7% |
Female 45.0% |
Missing 17.2% |
| Gestational agea (n = 114) |
Term 62.9% |
Preterm 12.6% |
Missing 24.5% |
| N (%) | |||
| Etiology (n = 151) | |||
| Hypoxic‐ischemic encephalopathy | 6 (4.0) | ||
| Cortical malformations | 3 (2.0) | ||
| CNS infections | 4 (2.6) | ||
| Metabolic disorders | |||
| Electrolyte imbalance | 3 (2.0) | ||
| Inborn errors of metabolism | 3 (2.0) | ||
| Vitamin‐related disorders | 11 (7.3) | ||
| Withdrawal seizures | 2 (1.3) | ||
| Genetics | |||
| Channelopathies | 67 (44.4) | ||
| Chromosomal disorders | 3 (2.0) | ||
| Other gene disorders | 7 (4.6) | ||
| Vascular | |||
| Stroke | 25 (16.6) | ||
| Hemorrhage | 8 (5.3) | ||
| Undetermined/unknown | 9 (6.0) | ||
| Seizure type (n = 151) | |||
| Sequential | 62 (41.1) | ||
| Clonic | 36 (23.8) | ||
| Tonic | 26 (17.2) | ||
| Autonomic | 14 (9.3) | ||
| Myoclonic | 9 (6.0) | ||
| Spasms | 3 (2.0) | ||
| Automatisms | 1 (0.7) | ||
| EEG (n = 151)b | |||
| Focal | 56 (37.1) | ||
| Burst‐suppression | 48 (31.8) | ||
| Multifocal | 46 (30.5) | ||
| Generalized | 1 (0.7) | ||
Information not available for all newborns.
Information related to ictal EEG except in some cases of burst‐suppression (BS). Burst‐suppression was described as an ictal pattern in 2 neonates and as an interictal in 8; in the remining cases, it was not clearly defined as an ictal or interictal pattern/background abnormality.
4.1. Etiology and seizure type
A genetic etiology was most frequently described among the cases included in this study (51.0%). This was followed by vascular (21.9%) and metabolic/vitamin‐related disorders (12.6%). In the genetic group, the most prevalent seizure type was sequential, described in 48.0% of the cases, followed by tonic seizures (33.7%; Table 3). It should be noted that a tonic component was reported in many sequential seizures, irrespective of the etiology. However, it was more frequently reported in genetic etiologies (64.2% of the cases; P = 0.019); it was reported in 12.5% of metabolic/vitamin‐related disorders. in 10.7% of vascular cases, in 8.93% of seizures of unknown etiology, in 1.7% of HIE, and in 1.7% of cortical malformations.
Table 3.
Seizures etiology × semiology
| Clonic | Tonic | Myoclonic | Automatisms | Spasms | Sequential | Autonomic | |
|---|---|---|---|---|---|---|---|
| Etiology/seizure classification, n (%) | |||||||
| HIE (n = 6) | 1 (16.7%) | 0 (0.0%) | 0 (0.0%) | 1 (16.7%) | 0 (0.0%) | 1 (16.7%) | 3 (50.0%) |
| Cortical malformations (n = 3) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (66.7%) | 1 (33.3%) |
| CNS infection (n = 4) | 3 (75.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (25.0%) | 0 (0.0%) |
| Metabolic disorders | |||||||
| Electrolyte imbalance (n = 3) | 1 (33.3%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (33.3%) | 1 (33.3%) |
| Inborn errors of metabolism (n = 3) | 0 (0.0%) | 0 (0.0%) | 3 (100%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| vitamin‐related disorders (n = 11) | 2 (18.2%) | 0 (0.0%) | 2 (18.2%) | 0 (0.0%) | 0 (0.0%) | 7 (63.6%) | 0 (0.0%) |
| Withdrawal (n = 2) | 1 (50.0%) | 0 (0.0%) | 1 (50.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Genetic disorders | |||||||
| Channelopathy (n = 67) | 5 (7.5%) | 26 (38.8%) | 3 (4.5%) | 0 (0.0%) | 0 (0.0%) | 33 (49.3%) | 0 (0.0%) |
| Chromosomal disorder (n = 3) | 1 (33.3%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (33.3%) | 1 (33.3%) |
| Other gene disorders (n = 7) | 2 (28.6%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (28.6%) | 3 (42.9%) | 0 (0.0%) |
| Vascular disorders | |||||||
| Stroke (n = 25) | 18 (72.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 5 (20.0%) | 2 (8.0%) |
| Hemorrhage (n = 8) | 2 (25.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (25.0%) | 4 (50.0%) |
| Unknown Undetermined/(n = 9) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (11.1%) | 6 (66.7%) | 2 (22.2%) |
CNS, central nervous system.
For the vascular etiology, the predominant seizure type was clonic (60.6%). For metabolic/vitamin‐related disorders, we found sequential seizures in 42.1% of the cases, followed by myoclonic in 31.5%.
Analysis of the relationship between seizure semiology and etiology revealed that sequential seizures occurred with all etiologies as shown in Table 3. Some seizure types significantly correlated with specific etiologies: infection and genetic disorders with clonic seizures (P = 0.042 and P < 0.001), metabolic/vitamin‐related disorders with myoclonic seizures (P < 0.001), and vascular with sequential seizures (P = 0.009). When specific etiologies were analyzed among the groups, certain etiologies were significantly associated with specific seizure types: hemorrhage with autonomic seizures (P = 0.003), stroke with clonic seizures (P < 0.001), and inborn errors of metabolism with myoclonic seizures (P < 0.001).
4.2. Etiology and EEG features
Certain etiologies were clearly related to specific EEG patterns. Focal ictal discharges were more prevalent in vascular etiologies (87.8%, P < 0.001), and burst‐suppression in genetic cases (51.9%, P < 0.001). Among the groups of etiologies, some specific disorders were also significantly correlated with ictal EEG patterns, such as electrolyte imbalance and focal discharges (100%, P = 0.049), vitamin deficiency and multifocal (63.3%, P = 0.035), and channelopathies and inborn errors of metabolism with burst‐suppression (50.7%, P < 0.001; and 100%, P < 0.001, respectively). Specific etiologies where the burst‐suppression pattern was described either as an ictal or interictal pattern are shown in Table 4.
Table 4.
Etiology vs EEG
| EEG n (%) | Generalized | |||
|---|---|---|---|---|
| Focal | Multifocal | Burst‐suppression | ||
| Etiology (n) | ||||
| Hypoxic‐ischemic encephalopathy (n = 6) | 4 (66.7%) | 2 (33.3%) | 0 (0.0%) | 0 (0.0%) |
| Cortical malformations (n = 3) | 2 (66.7%) | 1 (33.3%) | 0 (0.0%) | 0 (0.0%) |
| CNS infection (n = 4) | 1 (25.0%) | 3 (75.0%) | 0 (0.0%) | 0 (0.0%) |
| Metabolic/vitamins disorders (n = 19) | ||||
| Electrolyte imbalance (n = 3) | 3 (100%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Inborn errors of metabolism (n = 3) | 0 (0.0%) | 0 (0.0%) | 3 (100%)** | 0 (0.0%) |
| vitamin‐related disorders (n = 11) | 1 (9.1%) | 7 (63.6%) | 3 (27.3%)* | 0 (0.0%) |
| Withdrawal (n = 2) | 2 (100%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Genetic disorders | ||||
| Channelopathies (n = 67) | 10 (14.9%) | 22 (32.8%) | 34 (50.7%) | 1 (1.5%) |
| Chromosomal disorder (n = 3) | 0 (0.0%) | 3 (100%) | 0 (0.0%) | 0 (0.0%) |
| Other gene disorders (n = 7) | 1 (14.3%) | 0 (0.0%) | 6 (85.7%)** | 0 (0.0%) |
| Vascular disorders | ||||
| Stroke (n = 25) | 22 (88.0%) | 2 (8.0%) | 1 (4.0%) | 0 (0.0%) |
| Hemorrhage (n = 8) | 7 (87.5%) | 1 (12.5%) | 0 (0.0%) | 0 (0.0%) |
| Undetermined/unknown (n = 9) | 3 (33.3%) | 5 (55.6%) | 1 (11.1%)* | 0 (0.0%) |
CNS, central nervous system. Burst‐suppression was described as an ictal pattern *in 2 neonates (one with vitamin‐related disorder and one with unknown etiology) and as an interictal pattern **in eight (3 with inborn errors of metabolism, 3 with other gene disorders, and 2 with vitamin‐related disorders); in the remaining cases, it was not clearly defined as an ictal or interictal pattern/background abnormality.
4.3. Seizure type and EEG features
The predominant seizure type was sequential (41.1%), and the predominant EEG abnormality described was focal discharges (37.1%).
The frequency of each seizure type and related EEG features is presented in Table 5. Clonic seizures were mostly associated with focal ictal EEG abnormalities (61.1%, P = 0.001), and tonic and myoclonic seizures were associated with burst‐suppression (57.7%, P = 0.005; and 77.8%, P = 0.005). Autonomic seizures were also associated with focal EEG discharges in 85.7% of the cases (P < 0.001). The single case of automatisms was associated with a focal EEG discharge, and the 2 cases of epileptic spasms had a burst‐suppression pattern. Sequential seizures were equally associated with different EEG patterns (25.8% with focal, 45.2% with multifocal discharges, and 29.0% with burst‐suppression).
Table 5.
Seizure semiology x EEG
| Seizure semiology/EEG | ||||
|---|---|---|---|---|
| Focal | Multifocal | Generalized | Burst‐suppression | |
| Clonic (n = 36) | 22 (61.1%) | 8 (22.2%) | 1 (2.8%) | 5 (13.9%) |
| Tonic (n = 26) | 3 (11.5%) | 8 (30.8%) | 0 (0.0%) | 15 (57.7%) |
| Myoclonic (n = 9) | 2 (22.2%) | 0 (0.0%) | 0 (0.0%) | 7 (77.8%)* , ** |
| Automatisms (n = 1) | 1 (100%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Spasms (n = 3) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 3 (100%)** |
| Sequential (n = 62) | 16 (25.8%) | 28 (45.2%) | 0 (0.0%) | 18 (29.0%)** |
| Autonomic (n = 14) | 12 (85.7%) | 2 (14.3%) | 0 (0.0%) | 0 (0.0%) |
Burst‐suppression was described as an ictal pattern* in one neonate with myoclonic seizures and in one with spasms; as an interictal pattern** in 4 with myoclonic and four with sequential seizures; for the others it was not clearly defined as ictal or interictal pattern/background abnormality.
4.4. Emerging associations based on the report of etiology‐specific electroclinical features of neonatal seizures
We were able to establish some associations based on data acquired from this systematic review.
Some etiologies, generally related to acute events, were associated with specific clinical features and ictal EEG alterations, for example, 72% of the 25 patients with stroke had clonic seizures and 88% focal EEG; of the 4 patients with CNS infection, 3 had clonic seizures and multifocal EEG (75%).
Genetic and metabolic/vitamin‐related etiologies could also be associated with specific electroclinical features. The 3 cases of inborn errors of metabolism had myoclonic seizures and a burst‐suppression pattern described in all the patients. From the 11 patients with vitamin deficiency, 7 (63.6%) had sequential seizures and multifocal EEG findings. Among the 67 patients diagnosed with channelopathies, 88.0% presented either tonic or sequential seizures (involving a tonic component), 83.5% had a multifocal or burst‐suppression EEG, and 60 patients had mutations of the KCNQ2 gene. An analysis of this population showed specific combinations of semiology and EEG features: 25.0% had tonic seizures associated to burst‐suppression, 21.7% had sequential seizures with burst‐suppression, 20.0% had sequential seizures with a multifocal EEG, and 13.3% had tonic seizures and a multifocal EEG.
5. DISCUSSION
This systematic review aimed to establish a relationship between electroclinical features and etiology of neonatal seizures using existing studies published in the literature. The contribution of the present study to the extensive literature in this subject is the methodology applied. We have grouped the data of all the neonates from different authors and analyzed it as a large cohort, including specific information for each neonate. The observed associations may have a direct effect on clinical practice, mainly in institutions where continuous video‐EEG is not available or not obtainable at all times.
Continuous video‐EEG monitoring is essential for the accurate diagnosis of neonatal seizures. Previous studies have demonstrated that the extent of subclinical/electrographic seizures in neonates that can be missed in over 65% of seizures with only clinical detection.11, 14, 23, 24 However, in many countries and in population‐based studies, this technique is not readily available in all neonatal units.6, 7, 8, 9 We agree with previous reports that it is often difficult to accurately differentiate between seizure‐related and nonseizure movements in infants using clinical evaluation alone.11, 14, 23, 24 However, the combination of etiology, semiology, and EEG findings that we present in this review could help in classifying seizures in neonates.
We have observed that certain etiologies have a significant correlation with semiology (eg, stroke and CNS infection with clonic seizures, hemorrhage with autonomic seizures, inborn errors of metabolism, and the whole group of metabolic/vitamin‐related disorders with myoclonic seizures, channelopathies, and sequential or tonic seizures).
In contrast to previous studies of neonatal seizures where the predominant etiology was HIE,5, 6, 8, 9, 25, 26 we have observed an atypical distribution of etiologies, as the majority of cases with complete description of seizure semiology and EEG findings were related to a genetic etiology (either channelopathies or other gene disorders). This is likely due to a reporting bias, as a large number of case reports of genetic syndromes have appeared in the literature in the last few years. However, it is important to note that over the past several decades, the reported etiologies of neonatal seizures have significantly changed and that some etiologies (eg, hypocalcemia and other electrolytes imbalances)27, 28, 29 have decreased due to improved neonatal care. At the same time, the improvement and availability of genetic testing has led to more investigation of genetic etiologies of seizures and epilepsies in the neonate. This increased interest has led authors to focus their publications on the detailed description of these syndromes.30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41 In a recent paper, Shellhaas and collaborators reported the findings from “The National Seizure Registry” of 611 newborns. They observed a predominance of acute‐onset seizures (87.0%) in comparison to neonatal‐onset epilepsy. In those newborns epilepsy (n = 79), 46.8% had a genetic etiology and only 3.7% had HIE as a comorbidity.42
Because KCNQ2 mutations were the single most common cause of neonatal seizures identified in this review, we were able to confirm an electroclinical pattern highly suggestive of this diagnosis: sequential seizures (with a tonic component) or exclusively tonic seizures associated with a burst‐suppression or a multifocal EEG.
The seizure semiology description in the articles reviewed herein did not necessarily follow any of the previously proposed classifications.1, 5 Some authors simply described the observed motor phenomena in their own words and this made data comparison difficult to achieve. The Task Force on Neonatal Seizures, established by the ILAE in 2014, aimed to integrate current concepts in neonatal seizures and epilepsies into the 2017 ILAE Classification of Seizures and Epilepsies,17, 18 with a modification of this scheme, adapted to neonatal particularities. Widespread use of this proposal might be helpful for collecting data in future studies.
Some limitations of this study were related to issues of reliance on the quality of reported studies, unclear or incomplete description of seizure semiology, and inconsistent methods of reading and reporting EEG patterns. Many authors have used the term burst‐suppression to describe their EEG data without specifying if this pattern was consistent throughout the recording (and thus indicative of severe encephalopathy) or only during the motor seizure resembling an electrodecremental response. Future studies are needed to accurately describe the semiology of seizures that may be associated with an electrodecremental response or ictal burst‐attenuation. In addition, some authors did not specify the background activity or clearly differentiate ictal from interictal findings. We would recommend that a standardized reporting system for EEG studies, including description of the background activity, focality, as well as ictal and interictal patterns, be described to improve such systematic reviews. Due to the small percentage of preterm neonates in the sample, our findings might be more consistent for term neonates. Another limitation was that we were unable to develop a proportional meta‐analysis, since many of the studies reported fewer than 3 patients. Because of this, we had to group all neonates, as they belonged to one single study.19 On the other hand, this limitation gave us the opportunity to analyze all the data together as a large cohort.
Finally, due to reporting bias in the literature, we were not able to find papers describing electroclinical patterns for the most prevalent etiology of neonatal seizures, HIE, or any specific patterns characteristic for preterm neonates.
Recommendations for future studies may include the publication of complete clinical data of the neonates (including sex and gestational age) using a systematic approach to describe EEG findings and a consistent classification of neonatal seizures, using, for example, the proposed classification of neonatal seizures referenced by the ILAE Task Force17 when approved by the ILAE.
In conclusion, specific combinations of etiology, semiology, and EEG findings of neonatal seizures may be beneficial for an empirical approach to neonatal seizures. In this systematic review, we have shown that some etiologies have a specific correlation with semiology and ictal EEG patterns. These patterns may be helpful in making treatment decisions in countries with limited resources.
6. DISCLOSURE OF CONFLICTS OF INTEREST
Magda L Nunes was supported by CNPq‐Brazil, PQ grant 306338/2017‐3. Solomon L. Moshé was funded by grants from NIH U54NS100064 and NS43209, and from the Heffer Family, the Segal Family Foundations, and the Abbe Goldstein/Joshua Lurie and Laurie March/Dan Levitz families. Serves as an associate editor of Neurobiology of Disease and is on the editorial boards of Brain and Development, Pediatric Neurology, and Physiological Research. He receives from Elsevier an annual compensation for his work as associate editor of Neurobiology of Disease and royalties from books he coedited. He received a consultant fee from Mallinckrodt and UCB and is a member of a UCB Data Safety Monitoring Board (for work unrelated to this publication). Ronit M Pressler receives consultant fees from UCB (for work unrelated to this publication). The remaining authors have no conflicts of interest to declare. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Nunes ML, Yozawitz EG, Zuberi S, et al. Neonatal seizures: Is there a relationship between ictal electroclinical features and etiology? A critical appraisal based on a systematic literature review. Epilepsia Open. 2019;4:10–29. 10.1002/epi4.12298
REFERENCES
- 1. Mizrahi EM, Kellaway P. Characterization and classification of neonatal seizures. Neurology. 1987;37:1837–44. [DOI] [PubMed] [Google Scholar]
- 2. Mizrahi EM. Neonatal seizures: problems in diagnosis and classification. Epilepsia. 1987;28(suppl 1):S46–55. [DOI] [PubMed] [Google Scholar]
- 3. WHO . Guidelines on neonatal seizures. Geneva, Switzerland: World Health Organization; 2011. [PubMed] [Google Scholar]
- 4. Scher MS. Controversies regarding neonatal seizure recognition. Epileptic Disord. 2002;4:139–58. [PubMed] [Google Scholar]
- 5. Tekgul H, Gauvreau K, Soul J, et al. The current etiologic profile and neurodevelopmental outcome of seizures in term newborn infants. Pediatrics. 2006;117:1270–80. [DOI] [PubMed] [Google Scholar]
- 6. Garcias Da Silva LF, Nunes ML, da Costa JC. Risk factors for developing epilepsy after neonatal seizures. Pediatr Neurol. 2004;30:271–7. [DOI] [PubMed] [Google Scholar]
- 7. Ronen GM, Buckley D, Penney S, Streiner DL. Long‐term prognosis in children with neonatal seizures: a population‐based study. Neurology. 2007;69:1816–22. [DOI] [PubMed] [Google Scholar]
- 8. Kumar A, Gupta A, Talukdar B. Clinico‐etiological and EEG profile of neonatal seizures. Indian J Pediatr. 2007;74:33–7. [DOI] [PubMed] [Google Scholar]
- 9. Holanda MR, Melo AN. Comparative clinical study of preterm and full‐term newborn neonatal seizures. Arq Neuropsiquiatr. 2006;64:45–50. [DOI] [PubMed] [Google Scholar]
- 10. Pisani F, Piccolo B, Cantalupo G, et al. Neonatal seizures and postneonatal epilepsy: a 7‐y follow‐up study. Pediatr Res. 2012;72:186–93. [DOI] [PubMed] [Google Scholar]
- 11. Nagarajan L, Palumbo L, Ghosh S. Classification of clinical semiology in epileptic seizures in neonates. Eur J Paediatr Neurol. 2012;16:118–25. [DOI] [PubMed] [Google Scholar]
- 12. Nagarajan L, Ghosh S, Palumbo L. Ictal electroencephalograms in neonatal seizures: characteristics and associations. Pediatr Neurol. 2011;45:11–6. [DOI] [PubMed] [Google Scholar]
- 13. Pisani F, Barilli AL, Sisti L, et al. Preterm infants with video‐EEG confirmed seizures: outcome at 30 months of age. Brain Dev. 2008;30:20–30. [DOI] [PubMed] [Google Scholar]
- 14. Pisani F, Copioli C, Gioia CD, et al. Neonatal seizures: relation of ictal video‐encephalography (EEG) findings with neurodevelopmental outcome. J Child Neurol. 2008;23:394–8. [DOI] [PubMed] [Google Scholar]
- 15. Volpe JJ. Neonatal seizures: current concepts and revised classification. Pediatrics. 1989;84:422–8. [PubMed] [Google Scholar]
- 16. Scheffer IE, Berkovic S, Capovilla G, et al. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58:512–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Report of ILAE task Force on Neonatal Seziures [Cited 2018 Dec 10]. Available from https://www.ilae.org/guidelines/definition-and-classification/neonatal-seizure-classification
- 18. Fisher RS, Cross JH, D'Souza C, et al. Instruction manual for the ILAE 2017 operational classification of seizure types. Epilepsia. 2017;58:531–42. [DOI] [PubMed] [Google Scholar]
- 19. Moher D, Liberati A, Tetzlaff J, et al. Reprint‐Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Phys Ther. 2009;89:873–80. [PubMed] [Google Scholar]
- 20. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shea BJ, Grimshaw JM, Wells GA, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kelley GA, Kelley KS. Statistical models for meta‐analysis: a brief tutorial. World J Methodol. 2012;2:27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Murray DM, Boylan GB, Ali I, et al. Defining the gap between electrographic seizure burden, clinical expression and staff recognition of neonatal seizures. Arch Dis Child Fetal Neonatal Ed. 2008;93:F187–91. [DOI] [PubMed] [Google Scholar]
- 24. Malone A, Ryan CA, Fitzgerald A, et al. Interobserver agreement in neonatal seizure identification. Epilepsia. 2009;50:2097–101. [DOI] [PubMed] [Google Scholar]
- 25. Ronen GM, Penney S, Andrews W. The epidemiology of clinical neonatal seizures in Newfoundland: a population‐based study. J Pediatr. 1999;134:71–5. [DOI] [PubMed] [Google Scholar]
- 26. Gebremariam A, Gutema Y, Leuel A, et al. Early‐onset neonatal seizures: types, risk factors and short‐term outcome. Ann Trop Paediatr. 2006;26:127–31. [DOI] [PubMed] [Google Scholar]
- 27. Borkenhagen JF, Conner EL, Stafstrom CE. Neonatal hypocalcemic seizures due to excessive maternal calcium ingestion. Pediatr Neurol. 2013;48:469–71. [DOI] [PubMed] [Google Scholar]
- 28. Okumura A, Hayakawa F, Kato T, et al. Ictal electroencephalographic findings of neonatal seizures in preterm infants. Brain Dev. 2008;30:261–8. [DOI] [PubMed] [Google Scholar]
- 29. Gorman MP, Soul JS. Neonatal hypocalcemic seizures in siblings exposed to topiramate in utero. Pediatr Neurol. 2007;36:274–6. [DOI] [PubMed] [Google Scholar]
- 30. Pisano T, Numis AL, Heavin SB, et al. Early and effective treatment of KCNQ2 encephalopathy. Epilepsia. 2015;56:685–91. [DOI] [PubMed] [Google Scholar]
- 31. Saitsu H, Yamashita S, Tanaka Y, et al. Compound heterozygous BRAT1 mutations cause familial Ohtahara syndrome with hypertonia and microcephaly. J Hum Genet. 2014;59:687–90. [DOI] [PubMed] [Google Scholar]
- 32. Zerem A, Lev D, Blumkin L, et al. Paternal germline mosaicism of a SCN2A mutation results in Ohtahara syndrome in half siblings. Eur J Paediatr Neurol. 2014;18:567–71. [DOI] [PubMed] [Google Scholar]
- 33. Numis AL, Angriman M, Sullivan JE, et al. KCNQ2 encephalopathy: delineation of the electroclinical phenotype and treatment response. Neurology. 2014;82:368–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Weckhuysen S, Ivanovic V, Hendrickx R, et al. Extending the KCNQ2 encephalopathy spectrum:clinical and neuroimaging findings in 17 patients. Neurology. 2013;81:1697–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Serino D, Specchio N, Pontrelli G, et al. Video/EEG findings in a KCNQ2 epileptic encephalopathy: a case report and revision of literature data. Epileptic Disord. 2013;15:158–65. [DOI] [PubMed] [Google Scholar]
- 36. Mihl M, Boutry‐Kryza N, Sutera‐Sardo J, et al. Similar early characteristics but variable neurological outcome of patients with a de novo mutation of KCNQ2. Orphanet J Rare Dis. 2013;8:2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Allen NM, Mannion M, Conroy J, et al. The variable phenotypes of KCNQ‐related epilepsy. Epilepsia. 2014;55:e99–105. [DOI] [PubMed] [Google Scholar]
- 38. Kato M, Yamagata T, Kubota M, et al. Clinical spectrum of early onset epileptic encephalopathies caused by KCNQ2 mutation. Epilepsia. 2013;54:1282–7. [DOI] [PubMed] [Google Scholar]
- 39. Vatta M, Tennison MB, Aylsworth AS, et al. A novel STXBP1 mutation causes focal seizures with neonatal onset. J Child Neurol. 2012;27:811–4. [DOI] [PubMed] [Google Scholar]
- 40. Weckhuysen S, Mandelstam S, Suls A, et al. KCNQ2 encephalopathy: emerging phenotype of a neonatal epileptic encephalopathy. Ann Neurol. 2012;71:15–25. [DOI] [PubMed] [Google Scholar]
- 41. Blumkin L, Suls A, Deconinck T, et al. Neonatal seizures associated with a severe neonatal myoclonus like dyskinesia due to a familial KCNQ2 gene mutation. Eur J Pediatr Neurol. 2012;16:356–60. [DOI] [PubMed] [Google Scholar]
- 42. Shellhaas RA, Wusthoff CJ, Tsuchida TN, et al. Profile of neonatal epilepsies. Characteristics of a prospective US cohort. Neurology. 2017;89:893–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


