Figure 6.
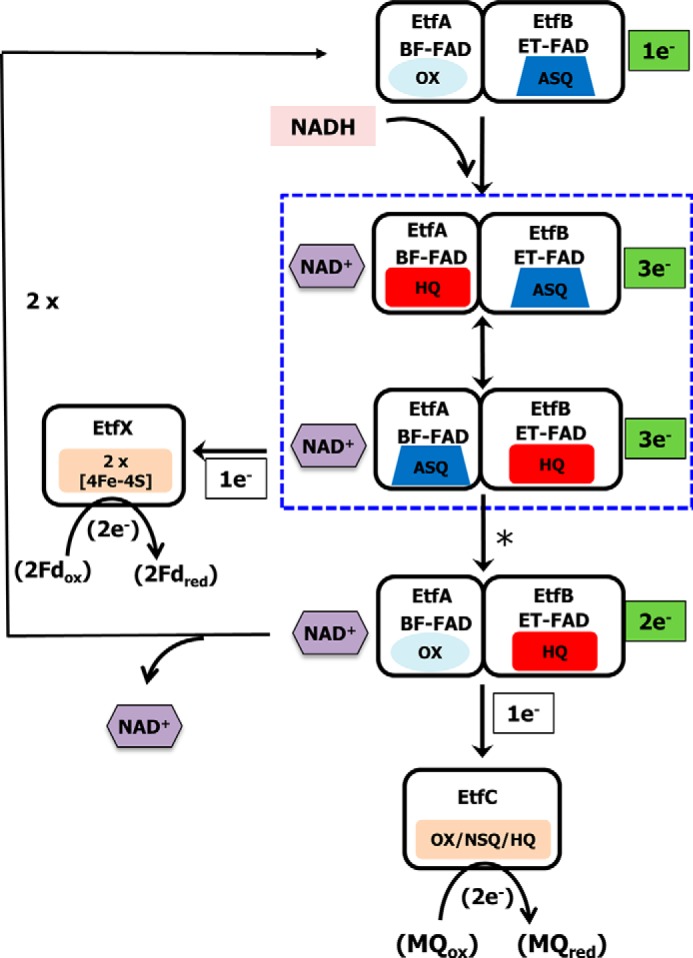
Proposed catalytic cycle of EtfABCX. The complex is depicted in its bifurcation-ready state in which the ET-FAD is in the ASQ state. In the first round of NADH oxidation, the BF-FAD is reduced to the HQ state and forms a CT complex with NAD+. The first electron is transferred to the ASQ-ET-FAD to generate the HQ state, leaving the ASQ-BF-FAD. The transfer of an electron to the low-potential branch [4Fe-4S] cluster triggers a protein conformational change (indicated by the asterisk) that then enables HQ-ET-FAD to reduce the FAD of EtfC with 1 e− converting it to the NSQ state. The catalytic cycle continues with the oxidation of a second NADH and the transfer of electrons down the low- and high-potential branches leading to the reduction of Fd and MQ, respectively. Boxed with a blue dotted line are the complexes with NAD+ that could form CT complexes.
