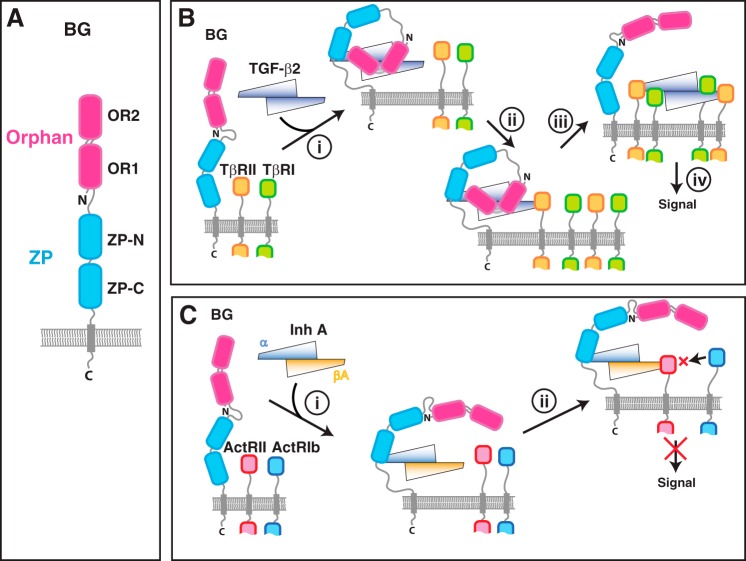
Figure 1.
Overall domain structure of betaglycan and proposed mechanisms for potentiation of TGF-β signaling and inhibition of activin signaling. A, overall domain structure of betaglycan. B, previously proposed mechanism for potentiation of TGF-β signaling by betaglycan (29) in which BG binds TGF-β dimers asymmetrically with a 1:1 stoichiometry in a manner that blocks one of the TβRII-binding sites but not the other (step i). Sequestration of TGF-β2 on the membrane by BG is proposed to promote binding of TβRII (step ii), which in turn promotes the concerted recruitment of TβRI and displacement of the BG orphan domain, and by an unknown mechanism, binding of a second TβRI:TβRII pair (step iii). C, previously proposed mechanism for inhibition of activin signaling by InhA (21, 23) in which BG binds InhA through its α-subunit (step i). Sequestration of InhA on the membrane by BG is proposed to promote binding of ActRII, which in turn sequesters ActRII away from activin A in a complex that is incapable of recruiting ActRIb and signaling (step ii).