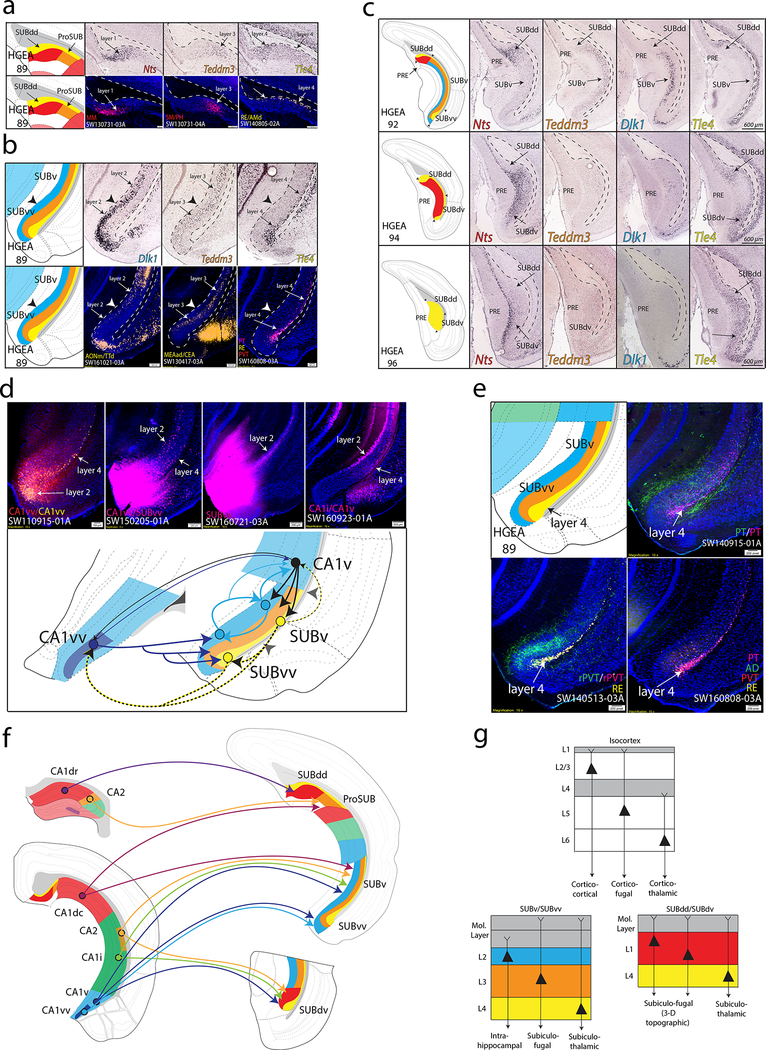
Figure 3. SUB gene expression and anatomical labeling patterns produce similar laminar organization.
(a) Gene expression patterns of the classic dorsal subiculum define laminar and subregional organization that correspond to anatomical connectivity patterns. Nts and Teddm3 expression demarcate HGEA layers 1 and 3, respectively, whereas Tle4 expression identifies the deeper HGEA layer 4 adjacent to the alveus. Below, example laminar retrograde labeling patterns of MM- (SW130731–03A), SM/PH- (SW130731–04A), and RE/AMd-projecting neurons (SW140805–02A) distinctly relate to layers 1, 3, and 4, respectively. (b) Teddm3 and Tle4 (layer 3 and 4) expression continues into the ‘classic’ ventral SUB, but both are located deeper to another layer identified by Dlk1 expression (layer 2). Note that there is a thickening of layer 4 at the dorsal and ventral ends of subiculum that we refer to as the dorsal and ventral ‘bulbs’, respectively. Below, example laminar retrograde labeling patterns of AONm/TTd- (SW161021–03A), MEAad/CEA- (SW130417–03A), and multiple thalamic-projecting neuronal cell types (PT, RE, PVT; SW160808–03A) distinctly relate to layers 2, 3, and 4, respectively. (c) At caudal levels, the distribution of the gene expression layers changes following the disappearance of the CA1. Immediately caudal to CA1 at ARA level 92, layers 2 and 3 extend dorsally to border layer 1. At ARA level 93, a ventral region containing layer 1 appears adjacent to the PAR (see Supplementary Fig. 1) and layer 1 becomes continuous at ARA level 94 following the emergence of the presubiculum (PRE). Finally, ARA level 96 represents the caudal end of the subiculum where only gene expression layer 4 is present (Nts expression is in PRE layer 3). The combinations and distribution of these layers are used to define the five SUB subregions. Scale bars on the right panels apply to all images. (d) Tracer coinjections within ventral CA1 and SUB regions revealed complex laminar-specific interconnectivity. CA1vv coinjection (SW110915–01A) produced anterograde labeling across all SUBvv layers and retrograde labeling within SUBvv layers 2 and 4. CTB injection into CA1vv/SUBvv (SW150205–01A) produced retrograde labeling within SUBv and SUBvv layer 4 and the deeper part of SUBv and SUBvv layer 2. PHAL anterograde injection into SUBvv (SW160721–03A) produced anterograde labeling that travels along SUBv and SUBvv layer 2. Retrograde injection into CA1v (SW160923–01A) produces retrograde labeling in SUBv layer 4 and superficial SUBv layer 2. Together, this data suggests that CA1v, CA1vv, SUBv, and SUBvv are bidirectionally connected through two different systems of connections mediated through layers 2 and 4 (see summary diagram below). (e) Coinjection into the paratenial thalamus (PT, SW140915–01A) produces anterograde and retrograde labeling within SUBv and SUBvv layers 3 and 4 as well as anterograde labeling in the SUBvv deep molecular layer. Coinjection into the rostral paraventricular thalamus (rPVT, SW140513–03A) and Fluorogold injection into the reuniens thalamus (RE) reveals strong retrograde labeling in SUBv and SUBvv layer 4 and anterograde labeling in SUBvv layer 3 and deep molecular layer. Multiple retrograde tracing from PT, PVT, RE, and anterodorsal thalamus (AD, SW160808–03A) produces retrograde labeling that is highly restricted to layer 4. (f) Summary schematic of CA1 and CA2 projections to the five SUB subregions. (g) General laminar organization of pyramidal neurons in the isocortex (top) compared similarly to the gene expression laminar organization of the SUBv/SUBvv and SUBdd/SUBdv pyramidal neurons. Extension of dendrites into the molecular layer is based on interpretation of rabies-labeled morphology (for example, see Figs. 5c and 6b). All in situ hybridization images in (a-c) are from Allen Institute website (www.brain-map.org). For the number of tracer experiments and cross-validated results, see the Supplementary Methods.