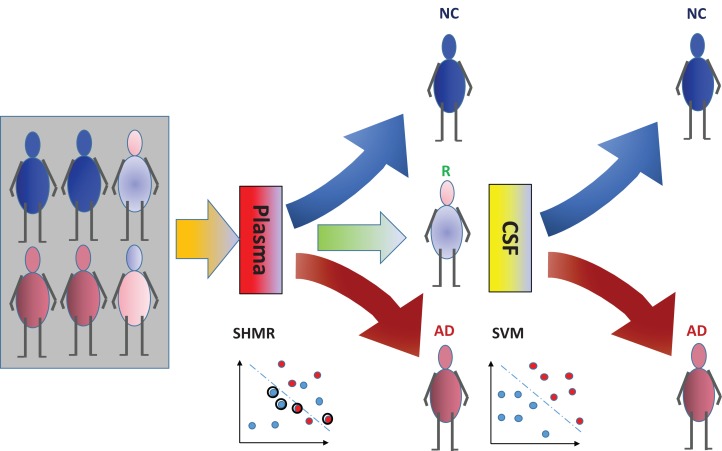
Figure 1. Cost-effective multistage framework for the diagnosis of Alzheimer’s disease (AD) patients from normal control (NC).
In a clinical setting, all registered patients can undergo an initial screening using inexpensive and easily accessible biomarkers (e.g., Plasma). Only those patients difficult to diagnosis (hence “Rejected: R” by SHIMR) are recommended for invasive and/or more expensive screening (e.g., CSF). Abbreviation: CSF, cerebrospinal fluid; SVM, support vector machines.