Figure 7.
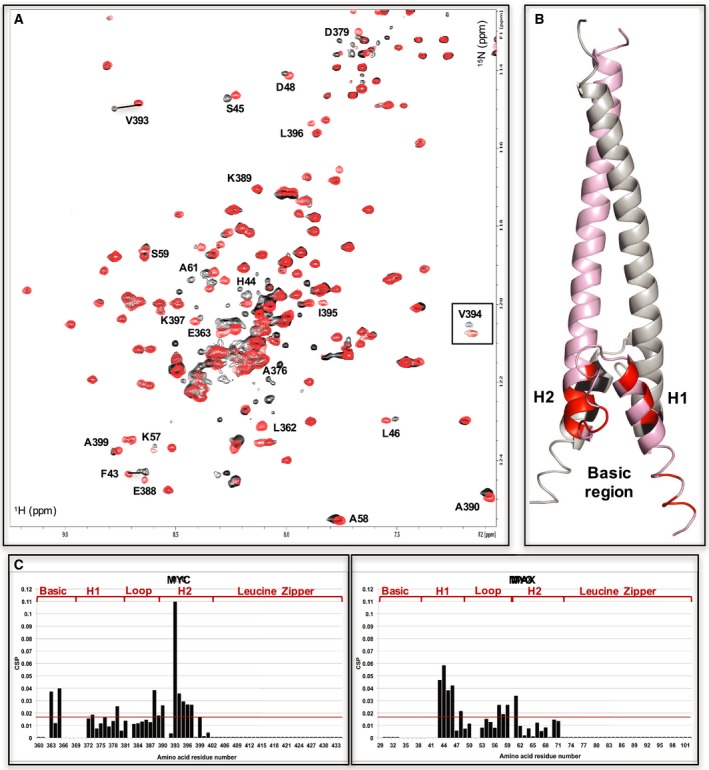
INI1/hSNF5 RPT1 binds to the helix‐loop‐helix region of MYC in the MYC:MAX bHLHZip dimer. (A) 1H,15N BST TROSY spectra of 15N‐labeled MYC:MAX bHLHZip dimer without (red) and with (black, ration 1 : 1) binding to RPT1 that move more than the standard deviation are labeled. The region corresponding to residue V394 is inserted in the spectra in a box to better illustrate the changes of chemical shifts that occur upon binding to INI1. (B) Cartoon representation of the MYC:MAX dimer (PDB = 1NKP, pink: MYC, gray: MAX) with highlighted in red (MYC) and black (MAX) the residues labeled in the spectra. H1 = MYC Helix 1; H2 = MYC Helix 2. (C) Diagrams showing the differences in chemical shifts induced by binding of RPT1 to the 15N‐labeled MYC:MAX dimer (MYC on the left, MAX on the right). Residues 29, 34–42, 52, 60, from MAX, and residues 366–71 from MYC are not assigned. L362 is assigned in the free form, but it could not be assigned in the bound form. Residues 51, 382, 391 are prolines. The red line indicates the standard deviation. H1 = MYC Helix 1; H2 = MYC Helix 2.
