Scheme 3.
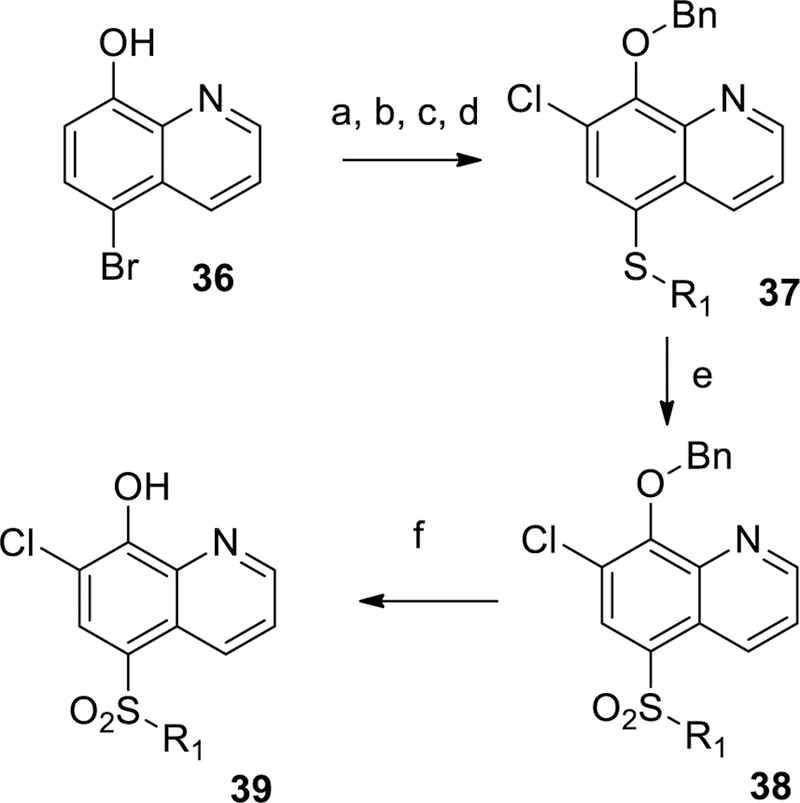
General synthetic procedures for 19, 22, and 26. Reagents and conditions: (a) NCS, CHCl3, 78%; (b) K2CO3, BnBr, ACN, 89%; (c) 2-trimethylsilylethanethiol (example 19) or R1SH (examples 22 and 26), Xantphos, Cs2CO3, Pd2(dba)3, dioxane, 100 °C, 93% example 19; 81% example 22, 93% example 26; (d) for example 19, 4-fluoroiodobenzene, TBAF, Xantphos, Cs2CO3, Pd2(dba)3, dioxane, 90 °C, 93%; (e) mCPBA, DCM; (f) 6M aqueous HCl, 100 °C.
