Abstract
Pembrolizumab, a humanized monoclonal antibody against programmed death 1 (PD‐1), has been shown to improve overall survival (OS) in patients with previously treated advanced non–small‐cell lung cancer (NSCLC) with programmed death ligand 1 (PD‐L1) tumor proportion score (TPS) ≥1%. We report safety and efficacy results from the phase 1b KEYNOTE‐025 study, which evaluated pembrolizumab in Japanese patients with previously treated NSCLC. Eligible patients had histologically/cytologically confirmed advanced NSCLC with PD‐L1 TPS ≥1% and had received ≥1 platinum‐doublet chemotherapy. Patients received pembrolizumab 10 mg/kg once every 3 weeks for 2 years or until disease progression/unacceptable toxicity. Primary objectives were to evaluate the safety of pembrolizumab in patients with PD‐L1 TPS ≥1% and the objective response rate (ORR) per RECIST version 1.1 in patients with PD‐L1 TPS ≥50%. Thirty‐eight patients were enrolled and received ≥1 pembrolizumab dose. The median (range) age was 66.0 (41‐78) years, and 61% had received ≥2 prior systemic therapies. Eleven patients (29%) experienced grade 3‐5 treatment‐related adverse events (AE); 9 patients (24%) experienced immune‐mediated AE and infusion reactions, with pneumonitis (11%; any grade) being most common. Among evaluable patients with PD‐L1 TPS ≥50% (n = 11), ORR was 27% (95% CI, 6‐61). Among evaluable patients with PD‐L1 TPS ≥1% (n = 37), ORR was 22% (95% CI, 10‐38). Median (95% CI) progression‐free survival and OS were 3.9 (2.0‐6.2) months and 19.2 (8.0‐26.7) months, respectively. In summary, pembrolizumab was generally well tolerated and showed promising antitumor activity in Japanese patients with previously treated PD‐L1–expressing NSCLC. Outcomes were consistent with those from the phase 3 KEYNOTE‐010 study. (Trial registration number: ClinicalTrials.gov, NCT02007070.)
Keywords: immunotherapy, non–small‐cell lung cancer, PD‐L1, pembrolizumab, phase 1
1. INTRODUCTION
In Japan, lung cancer accounts for approximately 20% of all cancer‐related deaths (approximately 77 200 in 2015) and is the leading cause of cancer‐related mortality in men (25% of all cancer deaths) and the second leading cause of cancer‐related mortality in women (14% of all cancer deaths).1 Standard‐of‐care therapy for patients with advanced non–small‐cell lung cancer (NSCLC) without sensitizing EGFR or ALK aberrations has typically comprised first‐line platinum‐based chemotherapy followed by single‐agent cytotoxic chemotherapy.2 Patients with sensitizing EGFR mutations or ALK aberrations may receive inhibitors targeting these molecules (ie, EGFR tyrosine kinase inhibitors and ALK inhibitors).2
The advent of immunotherapy has provided patients with NSCLC with treatment options that can significantly improve outcomes, with a manageable safety profile. Pembrolizumab is a highly selective, humanized monoclonal antibody against the programmed death 1 (PD‐1) receptor, which inhibits its interaction with its ligands, programmed death ligand 1 (PD‐L1) and 2.3 In the international phase 2/3 KEYNOTE‐010 study in patients with previously treated advanced NSCLC with a PD‐L1 tumor proportion score (TPS) ≥1%, pembrolizumab 2 mg/kg or 10 mg/kg every 3 weeks (Q3W) was shown to significantly improve overall survival (OS) compared with docetaxel and had a favorable benefit‐risk profile.4 Among patients with a PD‐L1 TPS ≥1%, hazard ratios (HR) for OS for pembrolizumab 2 mg/kg Q3W and 10 mg/kg Q3W versus docetaxel were .71 (95% CI, .58‐.88; P = .0008) and .61 (95% CI, .49‐.75; P < .0001), respectively. Among patients with PD‐L1 TPS ≥50%, HR (95% CI) for OS for pembrolizumab 2 and 10 mg/kg Q3W versus docetaxel were .54 (.38‐.77; P = .0002) and .50 (.36‐.70; P < .0001), respectively. In the first‐line setting, pembrolizumab 200 mg Q3W has been shown to improve OS and progression‐free survival (PFS) compared with platinum‐based chemotherapy in patients with advanced NSCLC with PD‐L1 TPS ≥50% without sensitizing EGFR or ALK aberrations in the phase 3 KEYNOTE‐024 study5 and to improve OS and PFS when combined with platinum‐pemetrexed compared with placebo plus platinum‐pemetrexed in the phase 3 KEYNOTE‐189 study6; in both studies, toxicity was manageable.
The phase 1b KEYNOTE‐025 study (ClinicalTrials.gov identifier, NCT02007070) was conducted in Japan and evaluated the efficacy and safety of pembrolizumab in patients with previously treated PD‐L1–expressing advanced NSCLC. Some recent evidence has suggested that efficacy and toxicity outcomes for Asian patients receiving systemic therapy for lung cancer may differ from those of Caucasian patients.7, 8 Herein, we report efficacy and safety outcomes from Japanese patients that received pembrolizumab in the KEYNOTE‐025 study.
2. METHODS
2.1. Eligibility
Patients ≥20 years old were eligible if they had a histologically or cytologically confirmed diagnosis of NSCLC with ≥1 measurable lesion as defined by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1,9 radiographic disease progression after treatment with a platinum‐based doublet chemotherapy for stage IIIB/IV or recurrent disease, radiographic disease progression while taking a tyrosine kinase inhibitor (erlotinib or gefitinib) for patients with EGFR sensitizing mutations or progressive disease while taking crizotinib for patients with ALK translocations, ≤2 prior systemic therapy regimens (3 if sensitizing EGFR mutations or ALK translocations are present), and an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. Eligible patients were also required to provide a newly obtained tumor tissue sample for analysis of PD‐L1 TPS, defined as the number of tumor cells with membranous PD‐L1 expression (evaluated as described below); only patients with a PD‐L1 TPS ≥1% were enrolled in the study. Patients were ineligible if they received systemic cytotoxic chemotherapy or biological therapy or had major surgery within 3 weeks of the first dose, received radiation therapy of >30 Gy within 6 months, received systemic steroid therapy within 3 days or were receiving any other immunosuppressive medication, had active central nervous system metastases (previously treated brain metastases were permitted if stable), had received any vaccine against infectious disease (eg, varicella and influenza) within 4 weeks, or had a history of or active autoimmune disease. Patients provided written informed consent before study participation. The protocol and all subsequent amendments were approved by an independent institutional review board or ethics committee at each study site. The study was conducted in compliance with Good Clinical Practice guidelines and the provisions of the Declaration of Helsinki.
2.2. Study design
KEYNOTE‐025 was an open‐label, nonrandomized, multicenter, phase 1b study of pembrolizumab in patients with PD‐L1–positive advanced NSCLC that was conducted in Japan. Pembrolizumab 10 mg/kg was administered intravenously Q3W over a 30‐minute infusion period. This dose was chosen based on data from KEYNOTE‐001, in which pembrolizumab 10 mg/kg Q3W had acceptable toxicity.10 Treatment continued for 2 years or until documented radiographic disease progression per immune‐related response criteria (irRC),11 unacceptable toxicity, intercurrent illness preventing further administration, investigator's decision, or withdrawal of consent.
The primary objectives of the study were to evaluate the objective response rate (ORR) per RECIST version 1.1 in patients with PD‐L1 TPS ≥50% and the safety and tolerability of pembrolizumab in patients with PD‐L1 TPS ≥1%. Secondary objectives included evaluation of ORR in patients with PD‐L1 TPS ≥1% and evaluation of duration of response (DOR), PFS per RECIST version 1.1, and OS in patients with PD‐L1 TPS ≥50% and ≥1%.
2.3. Assessments
Radiographic imaging (computed tomography or magnetic resonance imaging) was done at baseline and every 9 weeks thereafter. Response was assessed per irRC by investigators and per RECIST version 1.1 by independent radiologic review. After first documented disease progression per irRC, repeat imaging for confirmation was required ≥4 weeks later. Patients with confirmed radiographic progression (ie, 2 consecutive scans demonstrating progressive disease) were discontinued from trial treatment with exceptions considered for clinically stable or clinically improved patients. Tumor PD‐L1 status was evaluated from a newly obtained formalin‐fixed tumor tissue sample from a recent needle or excisional biopsy of a tumor lesion that had not been previously irradiated using the PD‐L1 IHC 22C3 pharmDx assay (Agilent Technologies, Carpinteria, CA, USA)12 at a central laboratory. Adverse events (AE) were monitored throughout the study, and patients were followed for up to 30 days after the end of treatment (up to 90 days for patients with serious and immune‐mediated AE and infusion reactions). AE were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.
2.4. Statistical analysis
It was determined that with 12 patients in the primary analysis population (patients with PD‐L1 TPS ≥50%), an assumed response rate of 50% and a 1‐sided significance level of .025, the study would have 80% power to demonstrate that the response rate was significantly greater than 15%. Because approximately half of the patients with PD‐L1 TPS ≥1% were expected to also have PD‐L1 TPS ≥50%, the target enrollment was 24 patients. Safety was evaluated in all patients who received ≥1 dose of pembrolizumab. Efficacy was evaluated in patients who received ≥1 dose of pembrolizumab and who had baseline radiographic imaging. PFS was defined as time from the first treatment to disease progression per RECIST version 1.1 or death from any cause, whichever occurred first. OS was defined as the time from the first treatment to death from any cause. DOR was defined as the time from the first documentation of a complete or partial response to disease progression. The objective response rate and its 95% confidence interval (CI) were calculated using the binomial exact method. Time‐to‐event endpoints (PFS, OS and DOR) were analyzed using the Kaplan‐Meier method. Patients without documented death at the time of analysis were censored at the time of last known contact.
3. RESULTS
3.1. Patients
Between March 2014 and September 2014, 78 patients were screened for PD‐L1 expression, of which 5 were not evaluable owing to lack of or insufficient tumor sample. Of the 73 patients with samples evaluable for PD‐L1, 47 had PD‐L1 TPS ≥1%. Thirty‐eight of these patients met all eligibility criteria and received ≥1 dose of pembrolizumab. At the time of data cutoff (31 July 2017), 3 patients (8%) had completed 2 years of treatment. In total, 33 patients (87%) discontinued treatment as a result of disease progression (n = 25; 66%), AE (n = 4; 11%), received medication not permitted per the study protocol (n = 2; 5%), death (n = 1; 3%) and physician decision (n = 1; 3%); study medication status was not recorded for 2 patients (5%). Median (range) duration of follow up was 19.2 (1.9‐34.6) months. Baseline characteristics are summarized in Table 1. Median (range) age was 66 (41‐78) years, 26 patients (68%) were male and 26 patients (68%) had an ECOG performance status of 1. Twenty‐three patients (61%) had received ≥2 prior systemic therapies, and 29 (76%) had adenocarcinoma histology.
Table 1.
Baseline demographic and disease characteristics
| Characteristic | All treated patients N = 38 |
|---|---|
| Median (range) age, y | 66 (41‐78) |
| <75 | 36 (95) |
| ≥75 | 2 (5) |
| Sex | |
| Male | 26 (68) |
| Female | 12 (32) |
| ECOG performance status | |
| 0 | 12 (32) |
| 1 | 26 (68) |
| Histology | |
| Adenocarcinoma | 29 (76) |
| Squamous cell carcinoma | 6 (16) |
| Adenosquamous | 2 (5) |
| Non–small‐cell NOS | 1 (3) |
| Metastatic stage | |
| M0 | 3 (8) |
| M1 | 1 (3) |
| M1A | 11 (29) |
| M2B | 23 (61) |
| Stable brain metastasis | 6 (16) |
| PD‐L1 TPS | |
| ≥50% | 12 (32) |
| 1%‐49% | 26 (68) |
| Smoking status | |
| Never | 13 (34) |
| Current/former | 25 (66) |
| Prior systemic therapies | |
| 1 | 15 (39) |
| 2 | 18 (47) |
| 3 | 5 (13) |
| EGFR mutation | |
| Mutant | 10 (26) |
| Wild type | 28 (74) |
| ALK translocation | |
| Mutant | 2 (5) |
| Wild type | 36 (95) |
Data are presented as n (%) unless otherwise indicated.
ECOG, Eastern Cooperative Oncology Group; NOS, not otherwise specified; PD‐L1, programmed death ligand 1; TPS, tumor proportion score.
3.2. Safety
Overall, 33 patients (87%) had a treatment‐related AE of any grade, with 11 (29%) experiencing ≥1 grade 3 to 5 treatment‐related AE (Table 2). The most frequently occurring treatment‐related AE were malaise (21%), diarrhea (16%) and maculopapular rash (16%). Nine patients (24%) had grade 3 treatment‐related AE; none occurred in >1 patient with the exception of decreased lymphocyte count (n = 2). Treatment‐related grade 4 and 5 AE were optic neuritis (n = 1; grade 4) and interstitial lung disease (n = 1; grade 5).
Table 2.
Patients with treatment‐related adverse events
| All treated patients, n (%) N = 38 | ||
|---|---|---|
| Any grade | Grades 3‐5 | |
| Treatment‐related AE | 33 (87) | 11 (29) |
| Treatment‐related AE occurring in >10% of patients | ||
| Malaise | 8 (21) | 0 (0) |
| Diarrhea | 6 (16) | 1 (3) |
| Rash maculopapular | 6 (16) | 0 (0) |
| Aspartate aminotransferase increased | 5 (13) | 1 (3) |
| Decreased appetite | 5 (13) | 1 (3) |
| Alanine aminotransferase increased | 5 (13) | 1 (3) |
| Pruritus | 5 (13) | 0 (0) |
| Rash | 5 (13) | 0 (0) |
| Nausea | 4 (11) | 0 (0) |
| Fatigue | 4 (11) | 0 (0) |
| Arthralgia | 4 (11) | 0 (0) |
| Immune‐mediated AE and infusion reactions, n (%)a | 9 (24) | 1 (3) |
| Colitis | 1 (3) | 0 (0) |
| Hyperthyroidism | 1 (3) | 0 (0) |
| Hypothyroidism | 2 (5) | 0 (0) |
| Infusion reactions | 1 (3) | 0 (0) |
| Myositis | 1 (3) | 0 (0) |
| Pneumonitis | 4 (11) | 1 (3) |
| Uveitis | 1 (3) | 0 (0) |
AE, adverse event.
Regardless of attribution to study treatment or immune‐relatedness by the investigator.
Immune‐mediated AE and infusion reactions (regardless of attribution to study treatment or immune relatedness by the investigator) occurred in 9 patients (24%; Table 2). The most frequently occurring of these were pneumonitis (n = 4; grade 5, n = 1), hypothyroidism (n = 2; both grade 1) and colitis (n = 2; includes grade 1 enterocolitis, n = 1; grade 2 colitis, n = 1). An independent radiologist and safety evaluation committee assessed the reported pneumonitis cases retrospectively and concluded that the fatal case of pneumonitis (grade 5 interstitial lung disease) was treatment‐related, whereas the other events (grade 2 interstitial lung disease; grade 2 pneumonitis; grade 2 organizing pneumonia) were not, based on clinical data and computed tomography findings. All 4 patients discontinued or interrupted pembrolizumab therapy. The case of grade 5 interstitial lung disease with a computed tomographic pattern of bilateral diffuse ground‐glass opacity occurred in a 61‐year‐old man after 2 doses of pembrolizumab (beginning 28 days after initiation of treatment). He was treated with pulse steroid therapy with methylprednisolone and then oral steroid tapering, but died because of worsening of interstitial lung disease (57 days after initiation of treatment). The remaining 3 events resolved.
Four patients discontinued treatment because of treatment‐related AE: there was 1 case of grade 2 myositis (onset at day 486; resolving at the data cutoff), 1 case of grade 4 optic neuritis (onset at day 104; not resolved) and 2 cases of interstitial lung disease (1 of grade 2 [onset at day 16; resolved after 17 days] and 1 of grade 5 [described above]).
3.3. Efficacy
This study did not meet the pre‐defined primary endpoint ORR criteria (ie, that the lower limit of the 95% CI for ORR among patients with PD‐L1 TPS ≥50% would be greater than 15%; see Methods). Among the 11 evaluable patients with PD‐L1 TPS ≥50%, 3 had a partial response and the confirmed ORR was 27% (95% CI, 6‐61); the lower limit of the 95% CI for ORR in this group was lower than the threshold of 15%. The median duration of response was not reached (range, 14.9‐29.1 [ongoing] months). Among the 37 patients with PD‐L1 TPS ≥1% who were evaluable for response, 8 had a partial response and none had a complete response (Table 3). The confirmed ORR was 22% (95% CI, 10‐38). Median duration of response was 22.8 months (range, 3.9‐29.1 [ongoing] months; Figure 1). Reductions in target lesion dimensions from baseline occurred in 27 of 37 patients (73.0%; Figures 2 and 3).
Table 3.
Best overall response assessed per RECIST version 1.1 central reviewa
| PD‐L1 TPS ≥50%, n = 11 | PD‐L1 TPS ≥1%, n = 37c | |||||
|---|---|---|---|---|---|---|
| n | % | 95% CIb | n | % | 95% CIb | |
| Objective response | 3 | 27 | 6‐61 | 8 | 22 | 10‐38 |
| Complete response | 0 | 0 | 0‐29 | 0 | 0 | 0‐10 |
| Partial response | 3 | 27 | 6‐61 | 8 | 22 | 10‐38 |
| Stable disease | 4 | 36 | 11‐69 | 12 | 32 | 18‐50 |
| Progressive disease | 4 | 36 | 11‐69 | 17 | 46 | 30‐63 |
CI, confidence interval; PD‐L1, programmed death ligand 1; RECIST, Response Evaluation Criteria in Solid Tumors, version 1.1; TPS, tumor proportion score.
Only confirmed responses are included.
Based on binomial exact CI method.
One patient who had no measurable lesion at baseline by central review was excluded from tumor response evaluation.
Figure 1.
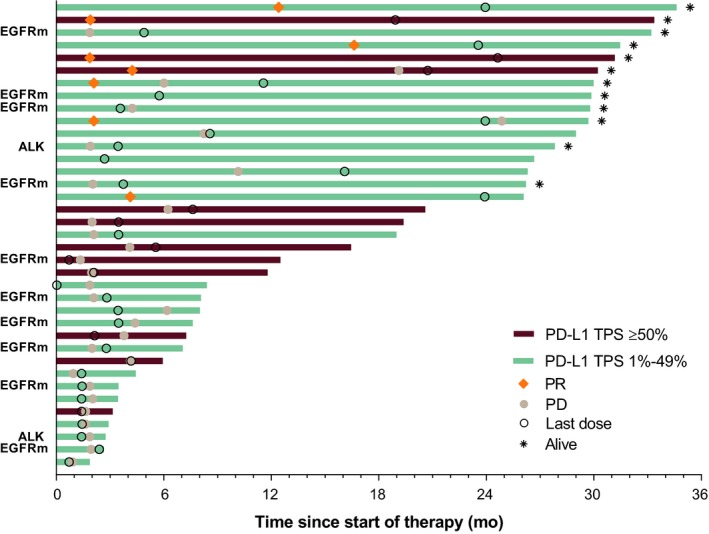
Treatment exposure, duration of response (as assessed per RECIST version 1.1 per central review), and overall survival time in individual patients. Bar lengths indicate time from start of first treatment to death or last follow‐up. ALK, anaplastic lymphoma kinase translocation; EGFRm, epidermal growth factor receptor mutant; PD, progressive disease; PD‐L1, programmed death ligand 1; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumors; TPS, tumor proportion score
Figure 2.
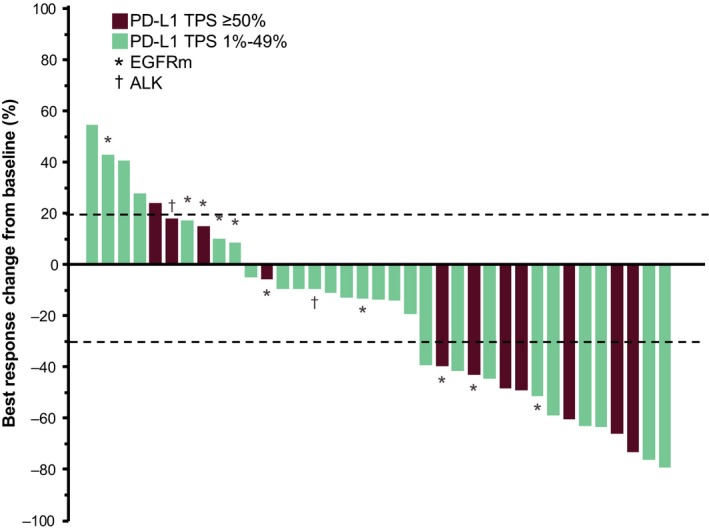
Best response change from baseline in tumor size for each patient as assessed per RECIST version 1.1 per central review. ALK, anaplastic lymphoma kinase translocation; EGFRm, epidermal growth factor receptor mutant; PD‐L1, programmed death ligand 1; RECIST, Response Evaluation Criteria in Solid Tumors; TPS, tumor proportion score
Figure 3.
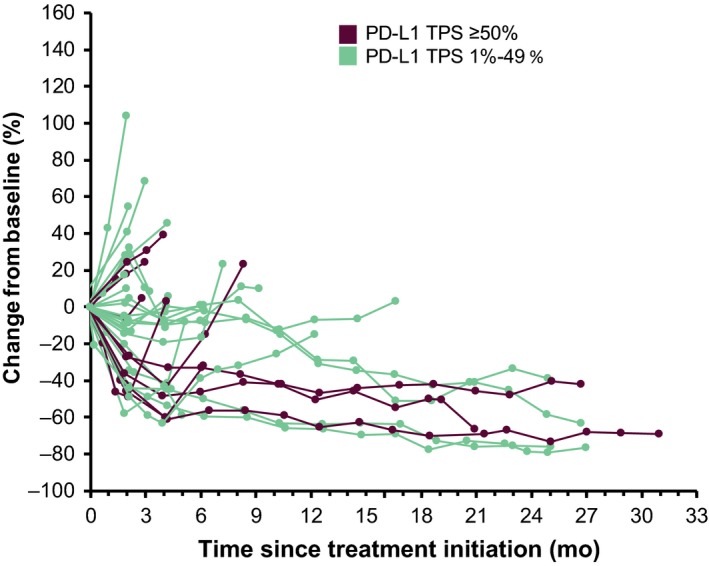
Longitudinal changes in the sum of the longest target lesion diameters for each patient as assessed per RECIST version 1.1 per central review. PD‐L1, programmed death ligand 1; RECIST, Response Evaluation Criteria in Solid Tumors; TPS, tumor proportion score
At the time of analysis, 10 of 12 (83%) patients with PD‐L1 TPS ≥50% had PFS events. The median (95% CI) PFS was 4.1 (1.6‐19.1) months, and the 6‐month PFS rate was 42% (Figure 4A). Thirty‐three of 38 (87%) patients with PD‐L1 TPS ≥1% had PFS events. The median (95% CI) PFS was 3.9 (2.0‐6.2) months, and the 6‐month PFS rate was 40%. Nine patients (75%) with PD‐L1 TPS ≥50% and 26 patients (68%) with PD‐L1 TPS ≥1% had died. The median (95% CI) OS in patients with PD‐L1 TPS ≥50% and TPS ≥1% were 17.9 (5.9–not reached) and 19.2 (8.0‐26.7) months, respectively. One‐year OS rates for patients with PD‐L1 TPS ≥50% and TPS ≥1% were 67% and 58%, respectively (Figure 4B).
Figure 4.
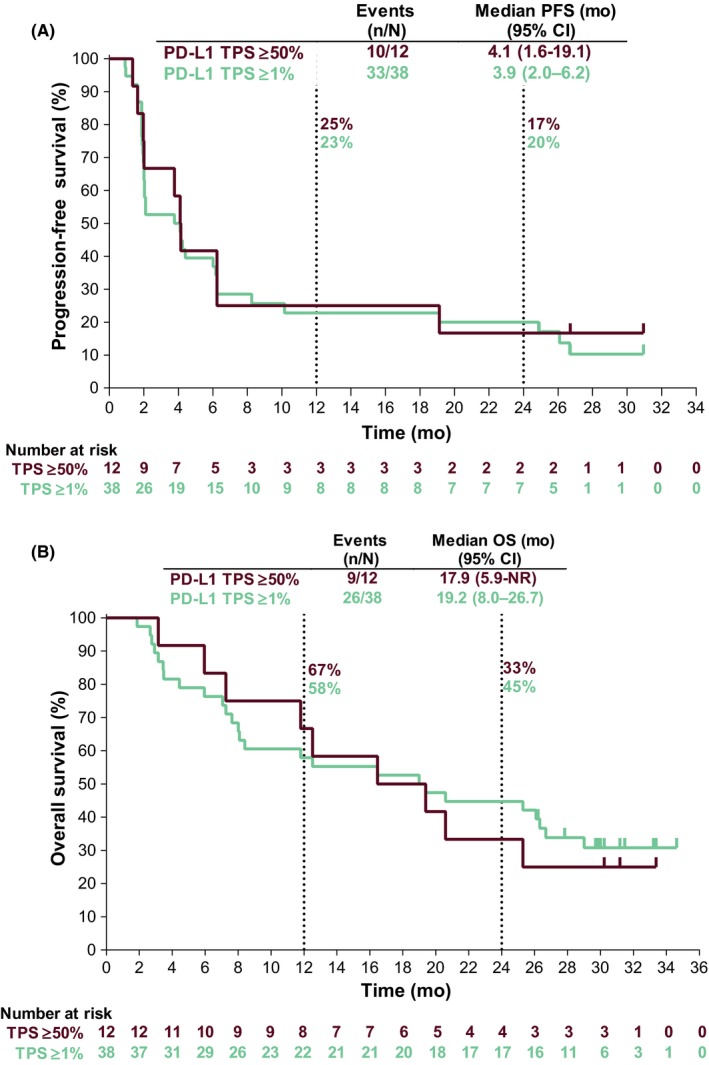
Survival estimates in patients who received pembrolizumab. (A) Progression‐free survival per RECIST version 1.1 per central review. (B) Overall survival. NR, not reached; OS, overall survival; PD‐L1, programmed death ligand 1; PFS, progression‐free survival; RECIST, Response Evaluation Criteria in Solid Tumors, version 1.1; TPS, tumor proportion score
Efficacy analyses in subgroups defined by select patient baseline characteristics are shown in Table S1. ORR was 15% (95% CI, 2‐45) among patients who had never smoked (n = 12) and 25% (95% CI, 10‐47) among former smokers (n = 26). ORR was similar in patients with adenocarcinoma histology (18% [95% CI, 6‐37]; n = 29) and squamous histology (17% [95% CI, .4‐64]; n = 6). Notably, among the 10 patients with EGFR mutations (all of whom had adenocarcinoma histology; Table S1) and the 2 patients with ALK translocations, none had a response (Figure 1). Among patients with wild‐type EGFR and adenocarcinoma histology (n = 19), the ORR was 29.6% (95% CI, 14‐50). In addition, the median PFS and OS appeared to be nominally shorter among patients with EGFR mutation versus adenocarcinoma patients without an EGFR mutation (Table S1).
4. DISCUSSION
In this phase 1b study, pembrolizumab 10 mg/kg once Q3W had promising antitumor activity and was well tolerated in Japanese patients with previously treated advanced NSCLC with a PD‐L1 TPS ≥1%. The safety profile of pembrolizumab in this study was generally similar to that reported in previous studies evaluating pembrolizumab monotherapy in patients with advanced NSCLC.4, 5, 10 The finding that pembrolizumab was well tolerated in Japanese patients is consistent with results from the KEYNOTE‐041 study, which found pembrolizumab to be well tolerated in Japanese patients with advanced melanoma.13 The most common treatment‐related AE in our study were malaise, diarrhea and rash. As in other studies evaluating pembrolizumab monotherapy in patients with NSCLC,4, 5, 10, 13 patients also experienced immune‐mediated AE and infusion reactions, including hypothyroidism and pneumonitis. Among the 4 patients (10.5%) who experienced pneumonitis, 3 had events of grade 2, and 1 had a fatal event. Only the grade 5 event was considered treatment‐related per the assessment of an independent radiologist and safety evaluation committee. Pneumonitis has been reported in other pembrolizumab NSCLC studies, albeit with lower incidence rates (any grade, 3.6%‐5.8%; grade 3‐5, 1.8%‐2.6%).4, 5, 10 Among Japanese patients enrolled in KEYNOTE‐010 (n = 62), 3 (4.8%) experienced pneumonitis of any grade, with 1 (1.6%) having a grade 3 event.14 In KEYNOTE‐041, which enrolled only Japanese patients with advanced melanoma (N = 42), 1 patient (2.4%) who received pembrolizumab experienced pneumonitis (grade 2).13 In a meta‐analysis,15 the incidence of pneumonitis of any grade among patients receiving anti–PD‐1 monotherapy was found to be 2.7% (95% CI, 1.9%‐3.6%; N = 4496). Given the number of patients enrolled in our study (N = 38), it is difficult to definitively determine whether pneumonitis events occurred more frequently in our Japanese study population. However, given the potential risks associated with this toxicity, it is important for physicians to recognize its symptoms and risk factors.16, 17
Treatment with pembrolizumab 10 mg/kg Q3W in this study was associated with clinically meaningful antitumor activity. Among patients with PD‐L1 TPS ≥50%, the ORR (95% CI) was 27.3% (6‐61), whereas among patients with PD‐L1 TPS ≥1%, the ORR was 22% (10‐38). Moreover, most patients (73.0%) had a reduction in tumor burden from baseline, and responses were durable; at data cutoff, the median duration of response had not been reached among patients who responded to therapy in the PD‐L1 TPS ≥50% group, and the response duration was 22.8 months for patients with a response in the PD‐L1 TPS ≥1% group. These outcomes are consistent with those reported in the international multicenter phase 2/3 KEYNOTE‐010 study4 and in the phase 1 KEYNOTE‐001 study (although it should be noted that KEYNOTE‐001 enrolled both previously treated and treatment‐naive patients).10 Although ORR in our study was nominally higher among the subgroup of patients with PD‐L1 TPS ≥50% versus the overall study population, there was little evidence of a difference in PFS or OS at these different PD‐L1 cutoffs. Moreover, median PFS and OS in our study were similar to those previously reported in the KEYNOTE‐010 study, both among patients with PD‐L1 TPS ≥1% and patients with PD‐L1 TPS ≥50%.4 In the first‐line setting, pembrolizumab 200 mg has been shown to improve OS, PFS and ORR compared with platinum‐based chemotherapy among patients with NSCLC with PD‐L1 TPS ≥50% (although it should be noted that these data are not directly comparable with those from this study owing to differences in study population).5
Subgroup analyses found that objective responses occurred across all patient subgroups defined by baseline tumor histology and smoking status. Notably, none of the patients with an EGFR mutation had an objective response. Some evidence has suggested that the presence of an EGFR mutation may be a negative predictive biomarker for OS treatment effect among patients with advanced NSCLC receiving anti–PD‐1 therapy.18 However, because KEYNOTE‐025 was a single‐arm study that enrolled only 10 patients with an EGFR mutation and did not include a comparator group, it was not possible to definitively evaluate this hypothesis in our study. Evaluation of response to checkpoint blockade in patients with EGFR mutations will require evaluation in larger studies.
Interestingly, in the phase 1 KEYNOTE‐001 study, no significant exposure dependency in efficacy and safety was identified for pembrolizumab across doses of 2 to 10 mg/kg.19 In addition, in the phase 3 KEYNOTE‐010 study in patients with previously treated advanced NSCLC with a PD‐L1 TPS ≥1%, pembrolizumab 2 mg/kg or 10 mg/kg Q3W showed a significant improvement in OS compared to docetaxel.4 This study used the highest dose of pembrolizumab (10 mg/kg, weight‐based dosing) employed in KEYNOTE‐001 and KEYNOTE‐010. Efficacy and safety results from this study in Japanese patients with previously treated NSCLC with PD‐L1 TPS ≥1% provide meaningful information for clinical practice in Japan. The currently approved dose for pembrolizumab for previously treated advanced NSCLC in Japan is a fixed dose (pembrolizumab 200 mg Q3W). Use of the fixed dose is supported by population pharmacokinetic modeling which found that both weight‐based and fixed‐dose regimens were appropriate for pembrolizumab and that fixed 200 mg Q3W dosing provided similar pharmacokinetic exposure to weight‐based 2 mg/kg dosing.20 Given that target saturation appears to occur with pembrolizumab doses greater than approximately 1 mg/kg,21, 22 outcomes in this study are unlikely to be different to those that would be achieved with the fixed dose.
In summary, pembrolizumab was generally well tolerated in Japanese patients with previously treated PD‐L1–positive advanced NSCLC, but pneumonitis should be carefully monitored. Pembrolizumab showed promising antitumor activity in this setting; outcomes were consistent with those from large phase 3 studies. These results support the use of pembrolizumab in Japanese patients with previously treated PD‐L1–positive advanced NSCLC.
CONFLICT OF INTEREST
Makoto Nishio: Honoraria from Ono Pharmaceutical, Bristol‐Myers Squibb, Pfizer, Chugai Pharmaceutical, Eli Lilly, Taiho Pharmaceutical, AstraZeneca, Boehringer Ingelheim, MSD, Novartis, Daiichi Sankyo, Merck Serono; grants from MSD, Novartis, Ono Pharmaceutical, Chugai Pharmaceutical, Bristol‐Myers Squibb, Taiho Pharmaceutical, Eli Lilly, AstraZeneca, Pfizer, Astellas. Toshiaki Takahashi: Honoraria from AstraZeneca K.K., Chugai Pharmaceutical, Eli Lilly Japan K.K.; grants from AstraZeneca K.K., Chugai Pharmaceutical, Eli Lilly Japan K.K., Ono Pharmaceutical, Pfizer Japan Inc., MSD K.K. Hiroshige Yoshioka: Honoraria from MSD, Chugai Pharmaceutical, Ono Pharmaceutical, Bristol‐Myers Squibb, AstraZeneca, Taiho Pharmaceutical, Pfizer, Eli Lilly, Boehringer Ingelheim. Kazuhiko Nakagawa: Honoraria from Eli Lilly Japan K.K., Novartis Pharma K.K., AstraZeneca, Pfizer Japan Inc., Astellas, Ono Pharmaceutical, Nippon Boehringer Ingelheim, MSD K.K.; grants from Chugai Pharmaceutical, Ono Pharmaceutical, Bristol‐Myers Squibb, Nippon Boehringer Ingelheim, Takeda Pharmaceutical, MSD K.K., EPS Associates, JCRO, Quintiles, PPD‐SNBL K.K., Pfizer Japan Inc., Kyowa Hakko Kirin, Astellas, PAREXEL International Corp., inVentiv Health Japan, ICON Japan K.K., A2 Healthcare Corp., AC Medical Inc. Tatsuro Fukuhara: Grants from MSD, Ono Pharmaceutical and Bristol‐Myers Squibb. Kazuhiko Yamada: No relevant disclosures. Masao Ichiki: No relevant disclosures. Hiroshi Tanaka: Honoraria from Boehringer Ingelheim, Bristol‐Myers Squibb, AstraZeneca, Chugai Pharmaceutical; grants from Taiho Pharmaceutical, AstraZeneca, Chugai Pharmaceutical, Astellas, Bristol‐Myers Squibb, Eli Lilly, MSD. Takashi Seto: Honoraria from AstraZeneca, Chugai Pharmaceutical, Eli Lilly, Nippon Boehringer Ingelheim, Ono Pharmaceutical, Pfizer, Taiho Pharmaceutical, Merck Sharp & Dohme Corp.; grants from Astellas, AstraZeneca, Chugai Pharmaceutical, Daiichi Sankyo, Eli Lilly, Eisai, Kissei Pharmaceutical, Merck Serono, Merck Sharp & Dohme Corp., Nippon Boehringer Ingelheim, Novartis, Pfizer, Verastem and Yakult Honsha. Hiroshi Sakai: Honoraria from Bristol‐Myers Squibb, Ono Pharmaceutical, MSD, Chugai Pharmaceutical, AstraZeneca; grants from Bristol‐Myers Squibb, Ono Pharmaceutical, MSD, Chugai Pharmaceutical, AstraZeneca, Merck Serono. Kazuo Kasahara: Grant from MSD. Miyako Satouchi: Honoraria from MSD, Chugai Pharmaceutical, Ono Pharmaceutical, Bristol‐Myers Squibb, AstraZeneca, Taiho Pharmaceutical, Pfizer, Novartis, Eli Lilly, Boehringer Ingelheim; grants from MSD, Chugai Pharmaceutical, Ono Pharmaceutical, Bristol‐Myers Squibb, AstraZeneca, Pfizer, Novartis, Boehringer Ingelheim, AbbVie, Takeda, Eli Lilly. Shi Rong Han: Employee of MSD K.K. Kazuo Noguchi: Employee of MSD K.K. Takashi Shimamoto: Employee of MSD K.K. Terufumi Kato: Advisory role for MSD, AstraZeneca, Boehringer Ingelheim, Chugai Pharmaceutical, Eli Lilly, Ono Pharmaceutical, Pfizer, Taiho Pharmaceutical and Sumimoto Dainippon; honoraria from MSD, AbbVie, AstraZeneca, Boehringer Ingelheim, Bristol‐Myers Squibb, Chugai Pharmaceutical, Eli Lilly, Kyowa Kirin, Novartis, Ono Pharmaceutical, Pfizer, Quintiles, Taiho Pharmaceutical, Roche and Merck Serono; grants from AbbVie, Astellas, AstraZeneca, Boehringer Ingelheim, Bristol‐Myers Squibb, Chugai Pharmaceutical, Eli Lilly, Kyowa Kirin, Novartis, Ono Pharmaceutical, Parexel, Quintiles, Taiho Pharmaceutical and Merck Serono.
Supporting information
ACKNOWLEDGEMENTS
We thank the patients and their families and caregivers for participating in this study, along with all investigators and site personnel. Medical writing and editorial assistance was provided by Mayur Kapadia, MD, of C4 MedSolutions, LLC (Yardley, PA, USA), a CHC Group company. This assistance was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
All authors are in agreement with the content of the manuscript, and have participated in the conception and design of the study, collection and assembly of data, or data analysis and interpretation; writing, and final approval of the manuscript.
Nishio M, Takahashi T, Yoshioka H, et al. KEYNOTE‐025: Phase 1b study of pembrolizumab in Japanese patients with previously treated programmed death ligand 1–positive advanced non–small‐cell lung cancer. Cancer Sci. 2019;110:1012–1020. 10.1111/cas.13932
Funding information
Funding for this research was provided by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. Pembrolizumab was provided by the study sponsor. Representatives from the study sponsor collected and analyzed the data.
REFERENCES
- 1. Center for Cancer Control and Information Services, National Cancer Center . Cancer statistics in Japan – 2015. 2016. https://ganjoho.jp/en/professional/statistics/brochure/2015_en.html. Accessed 3 March 2018.
- 2. Leighl NB. Treatment paradigms for patients with metastatic non‐small‐cell lung cancer: first‐, second‐, and third‐line. Curr Oncol. 2012;19:S52‐S58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Reck M. Pembrolizumab as first‐line therapy for metastatic non‐small‐cell lung cancer. Immunotherapy. 2018;10:93‐105. [DOI] [PubMed] [Google Scholar]
- 4. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non–small‐cell lung cancer (KEYNOTE‐010): a randomised controlled trial. Lancet. 2016;387:1540‐1550. [DOI] [PubMed] [Google Scholar]
- 5. Reck M, Rodriguez‐Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD‐L1‐positive non–small‐cell lung cancer. N Engl J Med. 2016;375:1823‐1833. [DOI] [PubMed] [Google Scholar]
- 6. Gandhi L, Rodgríguez‐Abreu D, Gadgeel S, et al. KEYNOTE‐189: randomized, double‐blind, phase 3 study of pembrolizumab (pembro) or placebo plus pemetrexed (pem) and platinum as first‐line therapy for metastatic NSCLC. American Association for Cancer Research Annual Meeting; 2018 April 14‐18; Chicago, IL, USA.
- 7. Soo RA, Kawaguchi T, Loh M, et al. Differences in outcome and toxicity between Asian and caucasian patients with lung cancer treated with systemic therapy. Future Oncol. 2012;8:451‐462. [DOI] [PubMed] [Google Scholar]
- 8. Nakanishi Y. Implementation of modern therapy approaches and research for non‐small cell lung cancer in Japan. Respirology. 2015;20:199‐208. [DOI] [PubMed] [Google Scholar]
- 9. Eisenhauer EA, Therasse P, Bogaerts J, et al. New Response Evaluation Criteria in Solid Tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228‐247. [DOI] [PubMed] [Google Scholar]
- 10. Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non–small‐cell lung cancer. N Engl J Med. 2015;372:2018‐2028. [DOI] [PubMed] [Google Scholar]
- 11. Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18:e143‐e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roach C, Zhang N, Corigliano E, et al. Development of a companion diagnostic PD‐L1 immunohistochemistry assay for pembrolizumab therapy in non‐small‐cell lung cancer. Appl Immunohistochem Mol Morphol. 2016;24:392‐397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yamazaki N, Takenouchi T, Fujimoto M, et al. Phase 1b study of pembrolizumab (MK‐3475; anti‐PD‐1 monoclonal antibody) in Japanese patients with advanced melanoma (KEYNOTE‐041). Cancer Chemother Pharmacol. 2017;79:651‐660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Saka H, Sugawara S, Hida T, et al. Japan subset analysis of phase III study of pembrolizumab for PD‐L1 positive, pretreated non‐small cell lung cancer (KEYNOTE‐010). Japan Lung Cancer Society; 2016.
- 15. Nishino M, Giobbie‐Hurder A, Hatabu H, Ramaiya NH, Hodi FS. Incidence of programmed cell death 1 inhibitor‐related pneumonitis in patients with advanced cancer: a systematic review and meta‐analysis. JAMA Oncol. 2016;2:1607‐1616. [DOI] [PubMed] [Google Scholar]
- 16. Sekine I, Yamamoto N, Nishio K, Saijo N. Emerging ethnic differences in lung cancer therapy. Br J Cancer. 2008;99:1757‐1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Delaunay M, Cadranel J, Lusque A, et al. Immune‐checkpoint inhibitors associated with interstitial lung disease in cancer patients. Eur Respir J. 2017;50 10.1183/13993003.00050-2017. [DOI] [PubMed] [Google Scholar]
- 18. Lee CK, Man J, Lord S, et al. Checkpoint inhibitors in metastatic EGFR‐mutated non‐small cell lung cancer–a meta‐analysis. J Thorac Oncol. 2017;12:403‐407. [DOI] [PubMed] [Google Scholar]
- 19. Chatterjee M, Turner DC, Felip E, et al. Systematic evaluation of pembrolizumab dosing in patients with advanced non‐small‐cell lung cancer. Ann Oncol. 2016;27:1291‐1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Freshwater T, Kondic A, Ahamadi M, et al. Evaluation of dosing strategy for pembrolizumab for oncology indications. J Immunother Cancer. 2017;5:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Patnaik A, Kang SP, Rasco D, et al. Phase I study of pembrolizumab (MK‐3475; anti‐PD‐1 monoclonal antibody) in patients with advanced solid tumors. Clin Cancer Res. 2015;21:4286‐4293. [DOI] [PubMed] [Google Scholar]
- 22. Elassaiss‐Schaap J, Rossenu S, Lindauer A, et al. Using model‐based “Learn and Confirm” to reveal the pharmacokinetics‐pharmacodynamics relationship of pembrolizumab in the KEYNOTE‐001 trial. CPT Pharmacometrics Syst Pharmacol. 2017;6:21‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


