Abstract
Background
COMT gene polymorphism is associated with mental disorders and sensitivity to pain. In this study, we investigated the association between the COMT gene polymorphism and labor anxiety and analgesia in pregnant women.
Subjects and methods
A total of 425 pregnant women undergoing labor analgesia were selected from May 2016 to February 2018. The COMT gene polymorphism was detected through the PCR with restriction fragment length polymorphism technique before childbirth. According to a COMT genotype, the enrolled pregnant women were subdivided into the Val/Val (allele GG) group, the Met/Met (allele AA) group, and the Val/Met (allele GA) group. Then, the intervertebral space of all pregnant women was injected with 3 mL of 2% lidocaine +6 mL of 0.08% ropivacaine and 6 µg of fentanyl. Labor analgesia was administered as follows: 80 mg of 0.08% ropivacaine +100 µg of fentanyl + normal saline to 100 mL. The general characteristics of the women were examined and recorded. In addition, the State Anxiety Inventory (SAI), VAS, Ramsay sedation score, and epinephrine and norepinephrine levels were compared and analyzed.
Results
A total of 391 pregnant women were enrolled in this study; among these pregnant women, there were 180 pregnant women in the GG group, 132 in the GA group, and 99 in the AA group. The minor allele frequency of COMT polymorphism among these pregnant women was 32.8%. Compared with the GG group, the SAI and VAS scores were higher, the Ramsay sedation score was lower, and the epinephrine and norepinephrine levels were higher in AA and GA groups (P<0.05). Nonetheless, there was no statistically significant difference in the SAI, VAS, Ramsay sedation score, and epinephrine and norepinephrine levels between the groups AA and GA (P>0.05).
Conclusion
The COMT gene polymorphism was associated with labor anxiety and analgesia among pregnant women, and the Val158Met mutation in the COMT gene could lead to worse labor anxiety and less-effective labor analgesia in pregnant women.
Keywords: COMT, polymorphism, labor anxiety, labor analgesia
Introduction
Anxiety and pain during childbirth are a common phenomenon and can lead to a change in hormone secretion (eg, adrenocorticotropic hormone, cortisol, catecholamines, and endorphin) and a decrease in pain threshold in pregnant women; in addition, aggravation of pain affects emotions.1 The above phenomena result in a fear–tension–pain syndrome and form a vicious circle for pregnant women. Eventually, these adverse factors lead to complications during childbirth, such as increased dystocia and cesarean section rates.2,3
Nowadays, research indicates that anxiety could activate contraction of the gallbladder, which in turn facilitates transmission of pain;4 in addition, some studies have revealed that a cholecystokinin antagonist can reduce the pain sensation caused by anxiety.5,6 Some studies have shown that thalamus is the pain center in human beings; besides, activities of the hippocampus, amygdala, frontal cortex, and prefrontal lobe in patients with anxiety are higher than those in healthy people, and the brain circuits activated by pain and anxiety overlap.7,8 Therefore, anxiety and pain are a pathological reaction that is not conducive to maternal and fetal adaptation in the childbirth environment.
Epidural analgesia is currently recognized as an effective way to relieve maternal labor pain, but there are individual differences in the efficacy.
With the completion of the Human Genome Project, the study of anxiety and pain treatments from a genetic perspective has attracted much attention. The enzyme catechol-O-methyltransferase (COMT) has a role in the processing of pain and may contribute to the variability in pain sensitivity. For the COMT enzyme, there are two major isoforms, differing by 50 amino acid residues at the N terminus, namely, membrane bound (MB-COMT) and soluble (S-COMT).9 Some studies have indicated that one single-nucleotide polymorphism (SNP; rs2097903) is located in the promoter region of MB-COMT, and the second SNP (rs6269) is located in the promoter region of S-COMT, and three SNPs (rs4633, rs4818, and rs4680) are located in the coding region of both S- and MB-COMT.10,11 SNP rs4680 is nonsynonymous and can cause substitution of valine (Val) with methionine (Met) at codon 158 (Val158Met),10,11 which could cause a three- to fourfold decrease in COMT activity, and lower COMT activity may lead to enhanced dopaminergic neurotransmission, with lower endogenous levels of enkephalins and thus exaggerated pain sensitivity.12 More specifically, another study has also shown that healthy volunteers with the Met/Met genotype have higher pain ratings than those with the Val/Val genotype.13 In addition, some studies suggest that the COMT gene polymorphism can affect human perception of pain by altering the activity of COMT, can regulate psychological and stress factors, and may become one of the genetic factors that determine individual differences in tolerance and responses to pain or other stressors.14,15 Finally, in patients with different diseases, anxiety and pain are often present too.16,17 Nonetheless, whether the COMT gene polymorphism could affect labor anxiety and analgesia in pregnant women remains unclear.
Therefore, based on the above observations, the purpose of this study was to investigate the association of the COMT gene polymorphism with labor anxiety and analgesia in pregnant women.
Subjects and methods
Study design, setting, and selection of patients
A total of 425 pregnant women undergoing labor analgesia in the Affiliated Chinese Medicine Hospital of the Southwest Medical University from May 2016 to February 2018 were selected. When these women were admitted to the hospital, they were included in this study. The pregnant women, who were in their final trimester and planning a vaginal birth of a single infant, were enrolled in the study (20–32 years old, 146–172 cm, 38–41 weeks of gestational age, I–II American Society of Anesthesiologists classification). The exclusion criteria were as follows: depression or another mental illness, cephalopelvic disproportion, a pathological obstetric condition, serious diseases related to internal medicine or surgery, a history of alcoholism and/or smoking, and contraindications of epidural anesthesia. The study protocol was approved by the medical ethics committee of the Affiliated Chinese Medicine Hospital of the Southwest Medical University, and written informed consent forms were signed by the pregnant women. This study was conducted in accordance with the Declaration of Helsinki and registration had been completed (No.: research registry 4488).
COMT detection and grouping of results on COMT
After the pregnant women were hospitalized, 3 mL of maternal peripheral venous blood was collected, placed in an anticoagulant tube, and stored in a −80°C freezer. According to the manufacturer’s protocol, DNA was extracted with a gene extraction kit (Tiangen Biochemical Technology Co., Ltd) and analyzed by the PCR with restriction fragment length polymorphism. The forward primer for the COMT polymorphism was 5′-ACTGGCTACTCAGCTGTG-3′ and the reverse primer was 5′-CCTTTTTCCAGGTCTGACAA-3′, which was synthesized by Shanghai Bioengineering Corporation (Shanghai, China). The PCR reaction system was as follows: 10× buffer (3 µL), dNTP mixture (0.5 µL), DNA template (2 µL), upstream and downstream primers (0.5 µL), Taq DNA polymerase (0.3 µL), the solution was made up to 30 µL with distilled water. PCR cycling conditions were as follows: 94°C for 5 minutes; then 35 cycles of denaturation at 94°C for 30 seconds, annealing at 55°C for 45 seconds, and extension at 72°C for 60 seconds; with final extension at 72°C for 10 minutes and storage at 4°C. The PCR was carried out on an ABI 3000 automatic DNA sequencing instrument (Thermo Fisher Scientific, Waltham, MA, USA), and the mutation was identified and typed by two-way sequencing. According to the genotype, the wild-type homozygote (Val/Val) was defined as GG, the mutant homozygote (Met/Met) was defined as AA, and the mutant heterozygote (Val/Met) was defined as GA.
Methods of labor analgesia
After regular uterine contraction, the pregnant women were given intravenous infusion of 500–1,000 mL of 0.9% normal saline. When the uterine orifice enlarged by 3–4 cm, epidural puncture in the intervertebral space L2–L3 was performed by an anesthesiologist. After successful puncture, the inter-vertebral space was injected with 3 mL of 2% lidocaine +6 mL of 0.08% ropivacaine and 6 µg of fentanyl; then, labor analgesia was administered via the same route: 80 mg of 0.08% ropivacaine +100 µg of fentanyl + normal saline to 100 mL and the background dose was 8 mL/h.
Observation indicators and evaluation criteria
Anxiety assessment
The pregnant women in the three groups were tested with the State Anxiety Inventory (SAI) by the same professionally trained and certified anesthesiologist. The requirements of the questionnaire were explained, and the pregnant women were asked to fill out the questionnaire. The higher the score, the more serious was the maternal anxiety; the normal range was <50 points, mild anxiety was denoted by 50–59 points, moderate anxiety by 60–69 points, and severe anxiety corresponded to >70 points.
Pain score
The pregnant women in three groups were administered the VAS by the same professionally trained and certified anesthesiologist. The requirements of the questionnaire were explained, and the pregnant women were asked to fill out the questionnaire. The higher the score, the more obvious was the pain; weak pain corresponded to <3 points, moderate pain to 4–6 points, and severe pain was denoted by 7–10 points.
Sedation score
The pregnant women in the three groups were evaluated via the Ramsay sedation score by the same anesthesiologist. The requirements of the questionnaire were explained, and the pregnant women were asked to fill out the questionnaire. The higher the score, the deeper was the sedation; an anxious state was denoted by 1 point, a quiet cooperation state corresponded to 2 points, a mild sleep state with waking up was denoted by 3 points, a moderate sleep state with a response to weak stimulation was denoted by 4 points, a moderate sleep state with a response only to strong stimulation was designated as 5 points, and a deep sleep state without waking up was denoted by 6 points.
Quantification of epinephrine and norepinephrine
The blood samples from pregnant women were collected at different time points and were immediately centrifuged; plasma aliquots were stored at −80°C until the analysis. According to the manufacturer’s protocol, the epinephrine and noradrenaline levels were determined by an ELISA Kit (Wuhan Huamei Biological Co., Ltd, Wuhan, China).
Statistical processing
All data were analyzed in SPSS 19.0 and GraphPad Prism 5.0 software. The data were presented as the mean ± SD or as n (%). One-way ANOVA and chi-squared test were used to evaluate the significance of differences in the general characteristics of pregnant women among the genotype groups. One-way ANOVA was conducted to analyze the SAI, VAS, and Ramsay sedation scores, and the epinephrine and norepinephrine levels in each group. A P-value <0.05 was assumed to represent a statistically significant difference.
Results
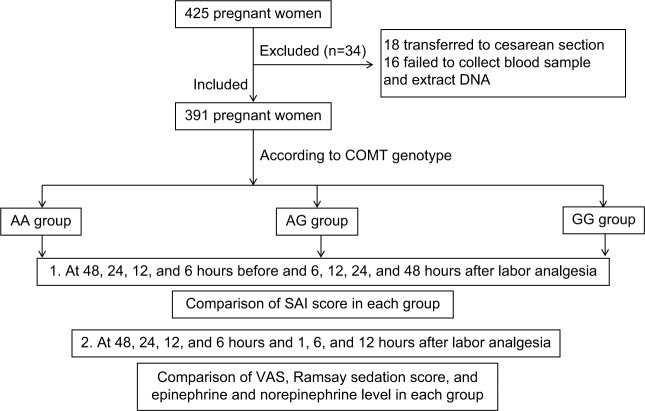
In this study, 425 pregnant women were selected; among these pregnant women, 18 pregnant women were subjected to cesarean section and 16 pregnant women failed to give a blood sample or extraction of DNA failed. Finally, 391 pregnant women were enrolled in this study, as shown in Figure 1.
Figure 1.
A flowchart of this study.
Abbreviation: SAI, State Anxiety Inventory.
COMT genotype and allele frequencies among pregnant women
Among the 391 pregnant women, there were 180 pregnant women in the GG group, 132 pregnant women in the GA group, and 99 pregnant women in the AA group. The allele distribution of COMT was found to be in line with the Hardy–Weinberg equilibrium (P>0.05). The minor allele frequency of SNP COMT was 32.8%, as shown in Table 1.
Table 1.
COMT genotypes and allele frequencies among pregnant women (%)
| COMT genotype | COMT allele mutation | |||
|---|---|---|---|---|
| GG | AA | GA | G | A |
| 46.0% | 20.3% | 33.7% | 67.2% | 32.8% |
Comparison of general characteristics of the pregnant women among the groups
There was no significant difference in age, weight, and gestational age among the three genotype groups (P>0.05), as shown in Table 2.
Table 2.
Comparison of general data of pregnant women among the groups
| Group | n | Agea (years) | Weighta (kg) | Heighta (cm) | Dysmenorrheab (%) | Gestationalb age (weeks) | Fibroidb (%) |
|---|---|---|---|---|---|---|---|
| GG group | 180 | 26.6±3.4 | 68.2±7.4 | 161.3±4.0 | 86 (47.8) | 39.4±1.1 | 4 (2.2) |
| AA group | 99 | 26.7±3.2 | 67.87±7.5 | 162.3±4.3 | 48 (48.4) | 39.3±1.5 | 4 (4.0) |
| GA group | 132 | 26.6±3.3 | 68.3±7.3 | 163.2±4.1 | 64 (48.5) | 39.2±1.8 | 4 (3.0) |
Notes: Data are presented as mean ± SD or n (%).
One-way ANOVA;
chi-squared test.
Comparison of SAI scores among the genotype groups
At 48, 24, 12, and 6 hours before labor analgesia and 6, 12, 24, and 48 hours after labor analgesia, the SAI was administered to assess anxiety among the pregnant women. The results showed that compared with the GG group, the SAI score was higher in groups AA and GA (P<0.05); however, there was no statistically significant difference in the SAI score between the AA group and GA group (P>0.05), as illustrated in Table 3.
Table 3.
Comparison of SAI score among the groups
| Group | 48 hours before | 24 hours before | 12 hours before | 6 hours before | 6 hours after | 12 hours after | 24 hours after | 48 hours after |
|---|---|---|---|---|---|---|---|---|
| GG group | 42±5 | 45±5 | 43±5 | 44±5 | 44±6 | 45±6 | 43±5 | 42±5 |
| AA group | 50±6* | 51±5* | 53±6* | 52±6* | 52±5* | 53±4* | 51±5* | 49±5* |
| GA group | 48±6* | 50±5* | 49±2* | 50±4* | 50±5* | 52±6* | 51±5* | 50±6* |
Note:
Compared with the GG group, one-way ANOVA; a P-value <0.05 was assumed to indicate statistical significance.
Abbreviation: SAI, State Anxiety Inventory.
Comparison of VAS scores among the groups
At 48, 24, 12, and 6 hours before labor analgesia and 1, 6, and 12 hours after labor analgesia, the VAS was employed to assess the pain in pregnant women. The results showed that compared with the GG group, the VAS score was higher in groups AA and GA (P<0.05); however, there was no statistically significant difference in VAS scores between the groups AA and GA (P>0.05), as shown in Table 4.
Table 4.
Comparison of VAS scores among the genotype groups
| Group | 48 hours before | 24 hours before | 12 hours before | 6 hours before | 1 hour after | 6 hours after | 12 hours after |
|---|---|---|---|---|---|---|---|
| GG group | 0.8±0.3 | 1.0±0.4 | 1.5±0.4 | 2.2±0.5 | 1.2±0.4 | 1.1±0.5 | 0.9±0.3 |
| AA group | 1.2±0.3* | 1.4±0.5* | 2.3±0.5* | 3.5±0.6* | 2.2±0.5* | 2.0±0.5* | 1.8±0.4* |
| GA group | 1.3±0.4* | 1.5±0.6* | 2.6±0.5* | 3.3±0.5* | 2.3±0.5* | 2.2±0.6* | 2.0±0.5* |
Note:
Compared with the GG group, one-way ANOVA; a P-value <0.05 was assumed to denote statistical significance.
Comparison of the Ramsay sedation score among the genotype groups
At 48, 24, 12, and 6 hours before labor analgesia and 1, 6, and 12 hours after labor analgesia, the Ramsay sedation score was determined to assess sedation in the pregnant women. The results revealed that compared with the GG group, the Ramsay sedation score was lower in groups AA and GA (P<0.05). Nevertheless, there was no statistically significant difference in the Ramsay sedation score between the groups AA and GA (P>0.05), as shown in Table 5.
Table 5.
Comparison of Ramsay sedation scores among the genotype groups
| Group | 48 hours before | 24 hours before | 12 hours before | 6 hours before | 1 hour after | 6 hours after | 12 hours after |
|---|---|---|---|---|---|---|---|
| GG group | 2.4±0.4 | 2.5±0.4 | 2.4±0.3 | 2.4±0.4 | 2.5±0.6 | 2.4±0.5 | 2.5±0.4 |
| AA group | 2.2±0.3* | 2.1±0.5* | 2.0±0.4* | 1.6±0.3* | 1.8±0.4* | 1.9±0.6* | 2.1±0.5* |
| GA group | 2.1±0.5* | 2.0±0.5* | 2.1±0.4* | 1.8±0.3* | 1.8±0.4* | 2.0±0.5* | 2.1±0.4* |
Note:
Compared with the GG group, one-way ANOVA; a P-value <0.05 was assumed to denote statistical significance.
Comparison of epinephrine and norepinephrine levels among the groups
At 48, 24, 12, and 6 hours before labor analgesia and 1, 6, and 12 hours after labor analgesia, ELISA was performed to quantify blood epinephrine and norepinephrine levels of pregnant women. The results indicated that compared with the GG group, the epinephrine and norepinephrine levels were higher in the groups AA and GA (P<0.05); however, there was no statistically significant difference in epinephrine and norepinephrine levels between the groups AA and GA (P>0.05), as presented in Tables 6 and 7.
Table 6.
Comparison of epinephrine levels among the genotype groups (ng/mL)
| Group | 48 hours before | 24 hours before | 12 hours before | 6 hours before | 1 hour after | 6 hours after | 12 hours after |
|---|---|---|---|---|---|---|---|
| GG group | 0.8±0.1 | 1.1±0.2 | 1.1±0.2 | 1.3±0.1 | 1.2±0.2 | 1.3±0.2 | 1.2±0.2 |
| AA group | 1.5±0.4* | 1.6±0.4* | 1.6±0.3* | 1.8±0.2* | 1.9±0.3* | 1.8±0.2* | 1.8±0.3* |
| GA group | 1.4±0.5* | 1.5±0.3* | 1.5±0.3* | 1.6±0.2* | 1.6±0.2* | 1.7±0.3* | 1.6±0.3* |
Note:
Compared with the GG group, one-way ANOVA; a P-value <0.05 was assumed to indicate statistical significance.
Table 7.
Comparison of norepinephrine levels among the genotype groups (ng/mL)
| Group | 48 hours before | 24 hours before | 12 hours before | 6 hours before | 1 hour after | 6 hours after | 12 hours after |
|---|---|---|---|---|---|---|---|
| GG group | 0.33±0.02 | 0.35±0.04 | 0.38±0.02 | 0.39±0.03 | 0.41±0.02 | 0.37±0.04 | 0.35±0.02 |
| AA group | 0.46±0.05* | 0.48±0.03* | 0.52±0.04* | 0.56±0.03* | 0.58±0.04* | 0.53±0.04* | 0.55±0.04* |
| GA group | 0.45±0.03* | 0.48±0.02* | 0.51±0.05* | 0.55±0.03* | 0.55±0.03* | 0.55±0.03* | 0.55±0.03* |
Note:
Compared with the GG group, one-way ANOVA; a P-value <0.05 was assumed to denote statistical significance.
Discussion
In this study, we, for the first time, found that the minor allele frequency of SNP COMT is 32.8% among pregnant women in China; this finding is similar to data obtained from a study in another country.18 Next, we investigated the association of the COMT polymorphism with labor anxiety and analgesia in pregnant women. The results revealed that compared with the GG group, the SAI and VAS scores were higher, Ramsay sedation score was lower, and epinephrine and norepinephrine levels were higher in groups AA and GA. This SNP can cause a substitution of valine (Val) with methionine (Met) at codon 158 (Val158Met); in addition, the wild-type homozygotes (Val/Val) were designated as the GG group, mutant homozygotes (Met/Met) as the AA group, and mutant heterozygotes (Val/Met) as the GA group. Therefore, the mutant COMT gene (Val158Met) could lead to worse labor anxiety and less-effective labor analgesia in pregnant women. Finally, we also found that the above findings were associated with epinephrine and norepinephrine levels in pregnant women. Therefore, the COMT gene polymorphism was found to be associated with labor anxiety and analgesia in pregnant women, and a mutated COMT allele could lead to worse labor anxiety and less-effective labor analgesia in pregnant women.
In this study, the SAI self-rating scale was employed to measure the anxiety in pregnant women. The results suggest that the anxiety score in groups AA and GA was significantly higher than that in the GG group, and the anxiety severity in groups AA and GA was significantly higher than that in the GG group. COMT, as the main enzyme degrading catecholamines, regulates the amount of catecholamines (and enkephalin) in vivo, including dopamine, norepinephrine, and adrenaline. Furthermore, the COMT Val158Met mutation can reduce the activity of COMT, thereby resulting in a decrease of catecholamine degradation and an increase in catecholamine amounts in vivo.19 Catecholamines are important hormones for a stress response and for adapting to the environment. The increase in catecholamine production can cause changes in the body that affect emotions and lead to short- and long-term stress-induced illnesses, such as psychiatric disorders, including anxiety.20
Labor analgesia is a widely used method for alleviation of labor pain, but its analgesic effect varies among different pregnant women. In this study, the results showed that the pain score in groups AA and GA was significantly higher than that in the GG group, whereas the sedation score in groups AA and GA was lower than that in the GG group. One study has revealed that the activity of COMT is an important regulator of pain sensitivity.21 Another study has also indicated that the Val158Met mutation of the COMT gene can affect the sensitivity of the body to pain by altering the activity of COMT and thus changing the hormone levels and the psychological state.22 In addition, the COMT gene polymorphism can cause a substitution of valine (Val) with methionine (Met) at codon 158 (Val158Met),10,11 which could result in a three- to fourfold decrease in COMT activity; lower COMT activity can increase the activity of dopaminergic neurotransmission, with lower endogenous levels of enkephalins, and thus may exaggerate pain sensitivity.12 More specifically, another study also indicates that healthy volunteers with the Met/Met genotype have higher pain ratings than those with the Val/Val genotype.13 In addition, some studies have shown that the COMT gene polymorphism can affect human perception of pain by altering the activity of COMT and by regulating psychological and stress factors and, therefore, may become one of the genetic determinants of individual differences in tolerance and response to pain or other stressors.
SNPs are the main cause of differences in pharmacological effects among individuals, meaning that the same drug is very effective for some people, but not for others or causes side effects.23,24 The Val158Met mutation of the COMT gene can reduce the activity of COMT; therefore, the degradation of catecholamines is decreased, and the amount of catecholamines will increase in vivo, and then reduce the levels of enkephalin, activate adrenergic receptors, increase the compensatory ability of opioid receptors, and increase the sensitivity of the body to opioid drugs.14,25 In the present study, we also found that the epinephrine and norepinephrine levels in genotype groups AA and GA are significantly higher than those in the GG group. This phenomenon is a possible mechanism via which the mutant COMT gene (Val158Met) could lead to worse labor anxiety and less-effective labor analgesia in pregnant women.
The advantage of this study lies in the retrospective analysis of the association between COMT gene polymorphism and labor anxiety and analgesia in pregnant women; it expands our knowledge about labor anxiety and analgesia in pregnant women. Nonetheless, there are some limitations to this study, such as a small sample size and a short period of evaluation of labor anxiety and analgesia. In addition, this study analyzed only a single mutation (Val158Met) of the COMT gene, without excluding the interference of related genes. Therefore, we still do not fully understand the effect of genes on labor analgesia, making it necessary to conduct a multimodal, multicenter study with a large sample size and a multigene combination related to analgesia.
Conclusion
COMT gene polymorphism was associated with labor anxiety and analgesia in pregnant women, and the Val158Met mutation in the COMT gene was associated with even worse labor anxiety and poorer effectiveness of labor analgesia in pregnant women.
Acknowledgments
Xiaohui Ren and Le Zhang are co-first authors.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Jokić-Begić N, Zigić L, Nakić Radoš S. Anxiety and anxiety sensitivity as predictors of fear of childbirth: different patterns for nulliparous and parous women. J Psychosom Obstet Gynaecol. 2014;35(1):22–28. doi: 10.3109/0167482X.2013.866647. [DOI] [PubMed] [Google Scholar]
- 2.Mazzoni A, Althabe F, Gutierrez L, et al. Women’s preferences and mode of delivery in public and private hospitals: a prospective cohort study. BMC Pregnancy Childbirth. 2016;16:34. doi: 10.1186/s12884-016-0824-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karlström A. Women’s self-reported experience of unplanned caesarean section: results of a Swedish study. Midwifery. 2017;50:253–258. doi: 10.1016/j.midw.2017.04.016. [DOI] [PubMed] [Google Scholar]
- 4.Colloca L, Benedetti F. Nocebo hyperalgesia: how anxiety is turned into pain. Curr Opin Anaesthesiol. 2007;20(5):435–439. doi: 10.1097/ACO.0b013e3282b972fb. [DOI] [PubMed] [Google Scholar]
- 5.Guszkowska M. The effect of exercise and childbirth classes on fear of childbirth and locus of labor pain control. Anxiety Stress Coping. 2014;27(2):176–189. doi: 10.1080/10615806.2013.830107. [DOI] [PubMed] [Google Scholar]
- 6.Angle P, Landy CK, Charles C, et al. Phase 1 development of an index to measure the quality of neuraxial labour analgesia: exploring the perspectives of childbearing women. Can J Anaesth. 2010;57(5):468–478. doi: 10.1007/s12630-010-9289-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mizuno A, Cherepanov SM, Kikuchi Y, et al. Lipo-oxytocin-1, a novel oxytocin analog conjugated with two palmitoyl groups, has long-lasting effects on anxiety-related behavior and social avoidance in CD157 knockout mice. Brain Sci. 2015;5(1):3–13. doi: 10.3390/brainsci5010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young P, Emery NC, Reisin R. Epidural analgesia for labor and delivery. N Engl J Med. 2010;363(4):395. [PubMed] [Google Scholar]
- 9.Diatchenko L, Slade GD, Nackley AG, et al. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum Mol Genet. 2005;14(1):135–143. doi: 10.1093/hmg/ddi013. [DOI] [PubMed] [Google Scholar]
- 10.Demille M, Kidd J, Ruggeri V, et al. Population variation in linkage disequilibrium across the Comt gene considering promoter region and coding region variation. Hum Genet. 2002;111(6):521–537. doi: 10.1007/s00439-002-0809-0. [DOI] [PubMed] [Google Scholar]
- 11.Xie T, Ho SL, Ramsden D. Characterization and implications of estrogenic down-regulation of human catechol-O-methyltransferase gene transcription. Mol Pharmacol. 1999;56(1):31–38. doi: 10.1124/mol.56.1.31. [DOI] [PubMed] [Google Scholar]
- 12.Zubieta JK, Heitzeg MM, Smith YR, et al. COMT Val158Met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science. 2003;299(5610):1240–1243. doi: 10.1126/science.1078546. [DOI] [PubMed] [Google Scholar]
- 13.Diatchenko L, Nackley AG, Slade GD, et al. Catechol-O-methyltransferase gene polymorphisms are associated with multiple pain-evoking stimuli. Pain. 2006;125(3):216–224. doi: 10.1016/j.pain.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 14.Finan PH, Zautra AJ, Davis MC, Lemery-Chalfant K, Covault J, Tennen H. COMT moderates the relation of daily maladaptive coping and pain in fibromyalgia. Pain. 2011;152(2):300–307. doi: 10.1016/j.pain.2010.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahlers SJ, Elens LL, van Gulik L, et al. The Val158Met polymorphism of the Comt gene is associated with increased pain sensitivity in morphine-treated patients undergoing a painful procedure after cardiac surgery. Br J Clin Pharmacol. 2013;75(6):1506–1515. doi: 10.1111/bcp.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brignardello-Petersen R. Anxiety related to dental treatment is probably associated with perceived pain, but the magnitude of this association remains unclear. J Am Dent Assoc. 2017;148(5):e54. doi: 10.1016/j.adaj.2017.02.038. [DOI] [PubMed] [Google Scholar]
- 17.Lin CS, Wu SY, Yi CA. Association between anxiety and pain in dental treatment: a systematic review and meta-analysis. J Dent Res. 2017;96(2):153–162. doi: 10.1177/0022034516678168. [DOI] [PubMed] [Google Scholar]
- 18.Mier D, Kirsch P, Meyer-Lindenberg A. Neural substrates of pleiotropic action of genetic variation in COMT: a meta-analysis. Mol Psychiatry. 2010;15(9):918–927. doi: 10.1038/mp.2009.36. [DOI] [PubMed] [Google Scholar]
- 19.Andersen S, Skorpen F. Variation in the Comt gene: implications for pain perception and pain treatment. Pharmacogenomics. 2009;10(4):669–684. doi: 10.2217/pgs.09.13. [DOI] [PubMed] [Google Scholar]
- 20.Kojima K, Ogomori K, Mori Y, Hirata K, Kinukawa N, Tashiro N. Relationship of emotional behaviors induced by electrical stimulation of the hypothalamus to changes in EKG, heart, stomach, adrenal glands, and thymus. Psychosom Med. 1996;58(4):383–391. doi: 10.1097/00006842-199607000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Comasco E, Hellgren C, Olivier J, et al. And COMT Val/Val genotype are associated with reduced sensorimotor gating in women. Psychoneuroendocrinology. 2015;60:217–223. doi: 10.1016/j.psyneuen.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 22.Qiu A, Tuan TA, Ong ML, et al. COMT haplotypes modulate associations of antenatal maternal anxiety and neonatal cortical morphology. Am J Psychiatry. 2015;172(2):163–172. doi: 10.1176/appi.ajp.2014.14030313. [DOI] [PubMed] [Google Scholar]
- 23.Sheikh HI, Kryski KR, Smith HJ, et al. Catechol-O-methyltransferase gene Val158Met polymorphism and depressive symptoms during early childhood. Am J Med Genet B Neuropsychiatr Genet. 2013;162B(3):245–252. doi: 10.1002/ajmg.b.32141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tammimäki A, Männistö PT. Catechol-O-methyltransferase gene polymorphism and chronic human pain: a systematic review and meta-analysis. Pharmacogenet Genomics. 2012;22(9):673–691. doi: 10.1097/FPC.0b013e3283560c46. [DOI] [PubMed] [Google Scholar]
- 25.Maeda K, Sugiyama Y. Impact of genetic polymorphisms of transporters on the pharmacokinetic, pharmacodynamic and toxicological properties of anionic drugs. Drug Metab Pharmacokinet. 2008;23(4):223–235. doi: 10.2133/dmpk.23.223. [DOI] [PubMed] [Google Scholar]