Fig. 1. (A) Diagram of the experimental design employing an osteoblast-osteoclast coculture system.
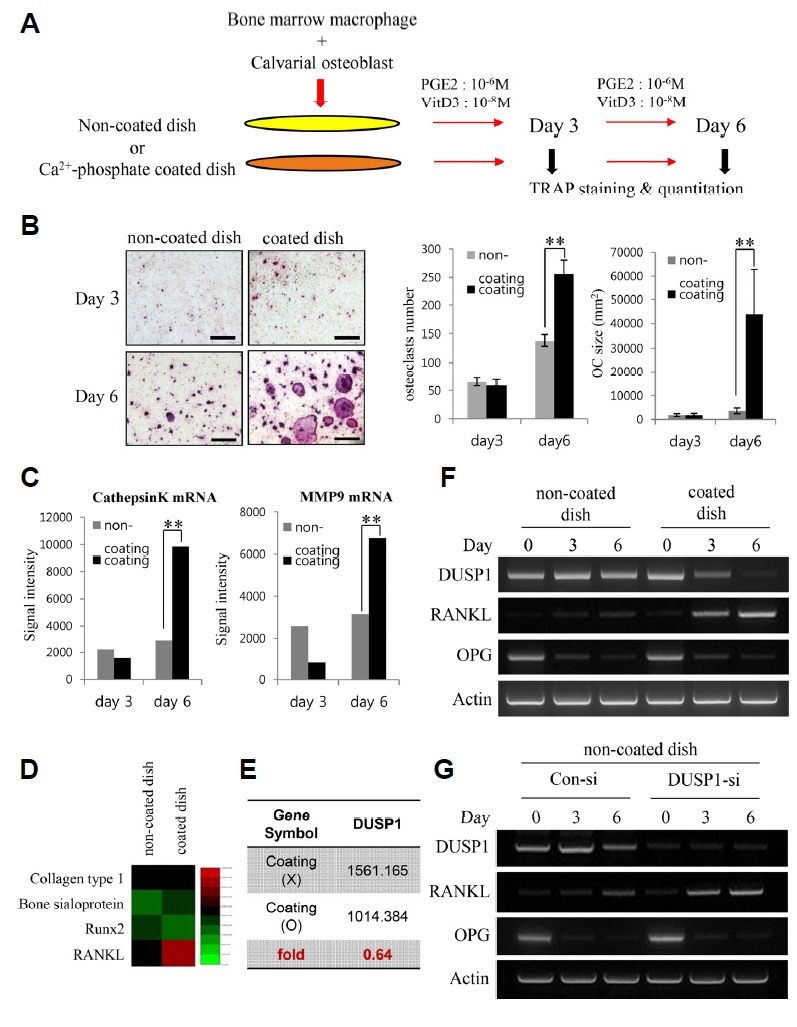
The cells were cultured for 3 or 6 days in the presence or absence of calcium-containing crystals on the surface of the wells. Prostaglandin E2 (PGE2) and vitamin D3 (VtD3) were added to stimulate osteoblast differentiation. The aim of the coculture was for cell surface RANKL on osteoblasts to support osteoclastic differentiation of BMMs. (B) TRAP staining of cells cultured in the non-coated and coated dish on days 3 and 6. There is a noticeable increase in the TRAP expression and number of multinucleated cells on day 6 of the coated dish. The number and average size (mm2) of TRAP-positive multinucleated osteoclasts were quantified. The data represent the means ± SD (**p < 0.01). (C) Quantification of Cathepsin K and MMP9 mRNA expression by qPCR. (D) Comparison of microarray heat map of genes associated with the differentiation of osteoblasts – Collagen type 1, Bone sialoprotein, and Runx2 – and osteoclasts – RANKL – between the uncoated and coated dish. The green and red colors indicate low and high expression, respectively. (E) Average signal value of DUSP1 gene expression in the uncoated and coated dishes evaluated from the microarray results. (F) RT-PCR analysis of DUSP1, RANKL, and OPG mRNA levels in the two dishes. DUSP1 levels were consistent in the non-coated dish. In the coated dish, the decrease in DUSP1 levels was associated with an increase in RANKL intensity. (G) RT-PCR analysis of the efficacy of DUSP1 knockdown and RANKL mRNA levels. Expression of RANKL increases in response to DUSP1 knockdown in the non-coated dish.
