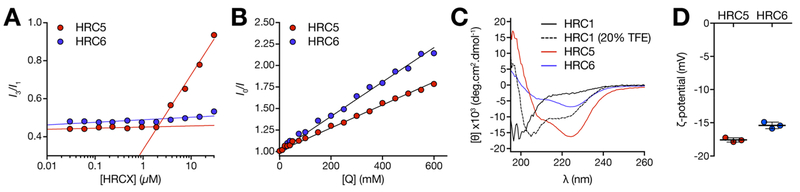
Figure 3.
Biophysical characterization of HRC5 and HRC6 NP structure. (A) HRC5 and HRC6 CMC determination through the pyrene 3:1 peak ratio method: pyrene (5 μM) fluorescence emission intensity ratio I3/I1 (I3, 383 nm; I1, 372 nm) was plotted as a function of the peptide concentration (0.03–30 μM). Lines correspond to linear regressions fitted to the experimental data regimes in the presence (high slope) and absence (low slope) of pyrene excimers. CMC is the x-axis value at the intersecion. Presented data sets are one of three independent replicates. (B) HRC5 and HRC6 NP internal accessibility probed by ANS fluorescence quenching. Stern−Volmer plots of ANS (12.8 μM) fluorescence emission intensity while inserted in HRC5 or HRC6 NP (30 μM), quenched by increasing acrylamide concentrations (0–600 mM). Lines correspond to the best fit of the Stern−Volmer equation (eq 1) to one of three independent replicates for each peptide. (C) HRC1, HRC5, and HRC6 peptide (30 μM) secondary structure in aqueous solution evaluated through CD spectroscopy. HRC1 was studied in aqueous solution and in the presence of 20% (v/v) TFE. Peptide mean residue ellipticity values ([θ]) were calculated according to eq 4. Presented spectra are one of three independent replicates. (D) HRC5 and HRC6 NP ζ-potential. Independent replicates correspond to the average of 15 measurements performed for each peptide sample (30 μM). Error bars correspond to the standard deviation of the mean.