Abstract
Purpose:
To test a pharmacist-led intervention to improve gout treatment adherence and outcomes.
Methods:
We conducted a site-randomized trial (n=1,463 patients) comparing a one-year, pharmacist-led intervention to usual care in gout patients initiating allopurinol. The intervention was delivered primarily through automated telephone technology. Co-primary outcomes were the proportion of patients adherent (proportion of days covered ≥0.8) and achieving a serum urate <6.0 mg/dl at one year. Outcomes were reassessed at year two.
Results:
Intervention patients were more likely than usual care patients to be adherent (50% vs. 37%; OR 1.68; 95% CI 1.30, 2.17) and reach serum urate goal (30% vs. 15%; OR 2.37; 95% CI 1.83, 3.05). In the second year (one year after the intervention ended), differences were attenuated, remaining significant for urate goal but not for adherence. The intervention was associated with a 6–16% lower gout flare rate during year two, differences not reaching statistical significance.
Conclusions:
A pharmacist-led intervention incorporating automated telephone technology improved adherence and serum urate goal achievement in gout patients initiating allopurinol. While this light-touch, low-tech intervention was efficacious, additional efforts are needed to enhance patient engagement in gout management and ultimately to improve outcomes.
Keywords: gout, allopurinol, serum urate, pharmacist, randomized trial, pragmatic trial, adherence
Gout is the most common form of inflammatory arthritis, affecting approximately 8 million individuals in the US1. Characterized by painful episodes of arthritis, gout results in substantial morbidity, disability, and mortality2–5. Contrary to other forms of arthritis, gout pathogenesis is well understood, with manifestations resulting from inflammatory responses triggered by monosodium urate crystal deposition that occurs when serum urate concentrations exceed 6.8 mg/dl6.
Recognizing hyperuricemia as a primary pathophysiologic culprit, urate-lowering therapy represents a cornerstone in gout management6. Allopurinol, the most common first-line urate-lowering agent, is efficacious and well-tolerated in most patients. Despite this, allopurinol use is frequently accompanied by suboptimal outcomes that stem from inadequate prescribing practices and poor adherence7–13. For gout patients initiating allopurinol, subspecialty management guidelines endorse gradual dose escalation to achieve a target serum urate14,15. These recommendations are based on evidence that maintaining serum urate concentrations <6.0 mg/dl reduces long-term flare risk14,16. Contrary to recommendations, most patients initiating allopurinol fail to undergo requisite laboratory assessments and only one in three receive dose increases10,17,18. Only a small fraction ever receive a daily dose >300 mg, dosing typically required to achieve desired urate concentrations and control of gout symptoms10,19.
To address barriers in achieving optimal treatment outcomes, we conducted the Randomized Evaluation of an Ambulatory Care Pharmacist-Led Intervention to Optimize Urate Lowering Pathways (RAmP-UP) study. RAmP-UP was designed to assess a highly automated, scalable, pharmacist-led, protocol-driven intervention to optimize allopurinol treatment in gout.
Methods and Materials
Study design, setting, randomization, and participants.
RAmP-UP was a large pragmatic site-randomized study that enrolled patients from May of 2014 to July of 2015. The study involved 116 clinics within Kaiser Permanente Southern California20, an integrated healthcare system with approximately 4 million members21. To minimize contamination, 24 close-proximity clinics were combined into nine units, resulting in 101 unique randomized study locations allocated to deliver either the intervention (n=51 sites) or usual care (n=50 sites). Stratified randomization was employed within hospital catchment areas to ensure balance in race/ethnicity. Given its pragmatic design, participant characteristics were collected only from electronic health records. As such, characteristics not routinely available in electronic records (e.g. age at first flare, results of crystal analysis, and the presence of other classification criteria such as tophi) were not collected.
Eligible patients were English-speaking, ≥18 years of age, with at least one International Classification of Disease, 9th edition diagnosis of gout (274.xx) and receiving a new allopurinol prescription. A new prescription was defined by ≥12 months of previous enrollment in the absence of a prescription. Patients with advanced chronic kidney disease (Stage V) were excluded. Eligible patients were sent an educational pamphlet and introductory letter. Patients and providers were given an opportunity to opt-out (occurring in <6% for both). We estimated that the part-time study pharmacist could deliver the intervention to a maximum of 15 patients/week. Thus, a second level of randomization was undertaken, generating a random list of up to 15 patients for enrollment each week.
Study procedures.
Following first allopurinol receipt, prescribed and initially dosed at the discretion of the primary gout provider, pharmacist management was provided to patients at intervention sites. We developed an intervention protocol to supplement usual care, which was modeled after an algorithm developed through expert consensus20. The algorithm incorporated a treat-to-target approach (including urate monitoring and allopurinol dose escalation). The intervention was delivered through an interactive voice response system22, used to assess whether patients continued to take prescribed treatment, alert patients regarding pending orders or prescriptions, and provide encouragement. Conditional on patients not refilling allopurinol prescriptions (assessed with pharmacy dispensing data), not undergoing required lab monitoring (available for review by the pharmacist), or not responding to automated messaging (with call completion data made available to the pharmacist in near-real time), the study pharmacist initiated telephone calls to patients to provide more in-depth assistance or respond to queries. Patients at non-intervention sites received usual care with the exception that all patients without a serum urate assessment in the previous 3 months received automated reminders at baseline and after one- and two-years of follow-up to undergo laboratory testing.
Study outcomes.
Co-primary outcomes were allopurinol adherence and achievement of a serum urate <6.0 mg/dl at one year. Adherence was assessed using pharmacy-dispensing data to calculate the proportion of days covered at one year with adherence defined as values ≥0.8. This is the preferred method for assessing medication adherence using pharmacy claims data 23. When multiple urate values were available, we used the value that was at least 7 days post-index and that was most proximate to one-year of follow-up. The proportion achieving serum urate <6.0 mg/dl at one year was examined in an additional subgroup analysis limited to adherent patients. The effect of treatment assignment on primary outcomes was examined in a separate subgroup analysis of “completers”, defined as intervention patients responding to at least one automated telephone survey.
Pre-specified secondary outcomes included: 1) urate goal achievement at year two; 2) allopurinol adherence during the second year; 3) absolute change in urate between baseline and follow-up and difference in absolute adherence at one year; 4) gout flares during the second year of observation; and 5) adverse events. Analysis focused on flares during year two as flare risk increases during initial urate lowering16. Flares were defined using an algorithm incorporating associated medical and/or pharmaceutical claims24 using electronic health data that was limited to the two-year observation period. Adverse events were captured through electronic health records and compiled retrospectively with an emphasis on diagnostic codes corresponding to severe cutaneous reactions (e.g. drug reaction with eosinophilia and systemic symptoms, erythema multiforme, Stevens-Johnson, or toxic epidermal necrolysis).
Analysis.
General estimating equations were used to examine group differences in primary outcomes in an intent-to-treat analysis, accounting for correlation among patients nested within site. Factors imbalanced by group following randomization (race and calendar year) were included as covariates. For dichotomous outcomes, non-responder imputation was used for models examining urate goal achievement when follow-up values were missing. A similar approach was used to examine the same dichotomous outcomes after two years of follow-up. Mixed effects linear regression was used to examine the continuous outcomes of absolute urate change and differences in adherence measures. Flare rates were calculated by group for each six-month interval of follow-up. The study had >90% power to detect a 10% difference in the proportion achieving a serum urate <6.0 mg/dl, assuming that 30% of patients receiving usual care would achieve this goal and an intra-class coefficient of 0.1 (“worst-case” for sample size planning).
Ethical considerations.
The study was approved by institutional review boards at Kaiser Permanente Southern California and at the University of Alabama at Birmingham. A waiver of informed consent was granted.
Results
There were 1,551 eligible patients initiating allopurinol with ICD-9 codes for gout (Figure 1). 782 (50.4%) received usual care at 50 clinics while 769 (49.6%) received care at intervention sites. There were 88 patients not offered an automated call following the second level of randomization, leaving 681 unique intervention patients for analysis (372 [55%] completers, responding to ≥1 automated survey). There were no significant differences in characteristics between the remaining 681 intervention patients and the 88 “untreated” patients from intervention sites (data not shown). Baseline characteristics of intervention and usual care patients are summarized in Table 1. Groups were similar with the exception that patients from intervention sites were more frequently Caucasian (45% vs. 38%; p=0.02) and more likely to initiate allopurinol in 2015 than in 2014 (46% vs. 41%; p=0.03).
Figure 1: Flow diagram summarizing design of the RAmP-UP study.
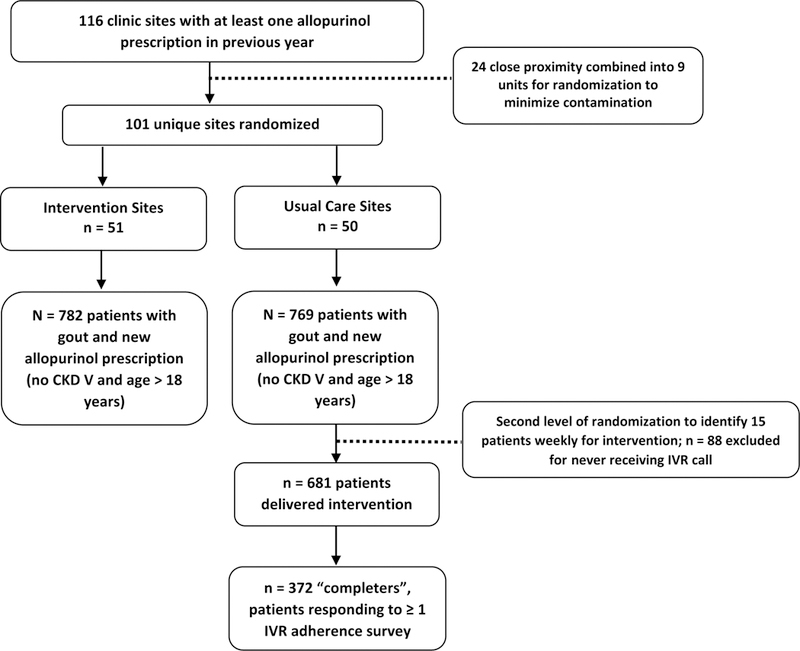
The study included 1,463 gout patients initiating allopurinol from 116 participating clinic sites from the Kaiser Permanente Southern California Health System.
Table 1:
Baseline participant characteristics
| Variable | Usual Care (n = 782) | Intervention (n = 681) |
|---|---|---|
| Age, mean (SD), years | 58.0 (14.4) | 58.6 (14.2) |
| Male Sex, % | 82.7 | 80.3 |
| Racea, % | ||
| American Indian / Eskimo | 0.4 | 0.4 |
| Asian | 26.0 | 19.8 |
| Black | 13.9 | 11.3 |
| Hawaiian or Pacific Islander | 4.9 | 4.6 |
| White | 37.9 | 45.2 |
| Unknown | 17.0 | 18.6 |
| Hispanic ethnicity, % | 23.3 | 25.7 |
| Body mass index, mean (SD), kg/m2 | 31.5 (6.5) | 31.5 (7.6) |
| Serum urate, mean (SD), mg/dl | 8.4 (1.6) | 8.4 (1.5) |
| Serum creatinine, mean (SD), mg/day | 1.16 (0.35) | 1.16 (0.35) |
| Allopurinol dose, mean (SD), mg/day | 188 (101) | 190 (98) |
| Allopurinol dose, mg/day, % | ||
| <100 | 0.9 | 0.6 |
| 100 to <200 | 50.1 | 48.9 |
| 200 to <300 | 14.1 | 12.9 |
| 300 | 33.1 | 36.9 |
| >300 | 1.8 | 0.7 |
| Index year of first allopurinol prescriptiona, % | ||
| 2014 | 59.3 | 53.6 |
| 2015 | 40.7 | 46.4 |
| Colchicine useb, % | 53.6 | 55.1 |
| Prescription NSAID useb, % | 49.2 | 51.4 |
| Glucocorticoid useb, % | 37.5 | 36.9 |
| Thiazide diureticb, % | 9.1 | 11.7 |
| Loop diureticb, % | 8.7 | 6.9 |
| Diabetes mellitusb, % | 21.7 | 23.1 |
| Hypertensionb, % | 57.8 | 59.8 |
Group differences examined using either chi square test or student‟s t-test; all comparisons were non-significant with the exception of race (p=0.020) and calendar year of first prescription (p=0.027)
Comorbidities and drug use defined using administrative diagnostic codes and pharmacy dispending data over the 12-month period preceding the first allopurinol prescription; NSAID = non-steroidal anti-inflammatory drug
Primary outcomes are shown in Table 2. Compared to usual care at one year, intervention patients were more adherent to allopurinol (50% vs. 37%, ORunadj 1.68; 95% CI 1.30, 2.17; p<0.001) and more likely to achieve a urate <6.0 mg/dl (30% vs. 15%, ORunadj 2.37; 95% CI 1.83, 3.05; p<0.001).
Table 2:
Achievement of serum urate (SU) goal and treatment adherence with intervention and usual care
| UC (n=782) | INT (n=681) | Unadjusted OR (95%CI) | P- value | Adjusted OR (95%CI) | P-value | |
|---|---|---|---|---|---|---|
| Primary outcomes (one-year) | ||||||
| SU <6.0 mg/dla | 15% | 30% | 2.37 (1.83–3.05) | <0.001 | 2.37 (1.84–3.06) | <0.001 |
| PDC ≥0.8a | 37% | 50% | 1.68 (1.30–2.17) | <0.001 | 1.68 (1.30–2.16) | <0.001 |
| Subgroup analyses (one-year) | ||||||
| SU <6.0 mg/dl in completersb | 15% | 33% | 2.70 (2.02, 3.61) | <0.001 | 2.61 (1.95, 3.51) | <0.001 |
| PDC ≥0.8 in completersb | 37% | 56% | 2.11 (1.61, 2.78) | <0.001 | 2.04 (1.56, 2.67) | <0.001 |
| SU <6.0 mg/dl in those with PDC ≥0.8 | 24% | 38% | 1.88 (1.27–2.77) | <0.001 | 1.94 (1.30–2.90) | <0.001 |
| Secondary outcomes (two-year) | ||||||
| SU <6.0 mg/dl | 23% | 31% | 1.45 (1.15–1.82) | 0.001 | 1.43 (1.14–1.80) | <0.001 |
| PDC ≥0.8 | 32% | 39% | 1.31 (1.00–1.73) | 0.05 | 1.29 (0.99–1.69) | 0.06 |
| SU <6.0 mg/dl in those with PDC ≥0.8 | 38% | 41% | 1.07 (0.75–1.51) | 0.71 | 1.07 (0.75–1.51) | 0.71 |
SU = serum urate, PDC = proportion of days covered
completer analysis limited to intervention patients completing at least 1 interactive voice recognition (IVR) adherence assessment
In analyses limited to adherent patients, the impact of the intervention on urate goal achievement at one year was slightly attenuated although significant (ORunadj 1.88; 95% CI 1.27, 2.77). In additional analyses, intervention patients completing ≥1 automated call were significantly more likely than patients receiving usual care to be adherent (56% vs. 37%; ORunadj 2.11; 1.61, 2.78) and to achieve urate goal (33% vs. 15%; ORunadj 2.70; 95% CI 2.02, 3.61) at one year. Results were unchanged following adjustment for race and calendar year (Table 2).
Following termination of the intervention at 1 year, group differences in adherence during the second year (ORunadj 1.31; 95% CI 1.00, 1.73) and urate goal attainment at two years (ORunadj 1.45; 95% CI 1.15, 1.82) were attenuated compared to year one with differences in adherence no longer achieving significance following adjustment (p=0.06). In analyses limited to adherent patients after one year, the odds of urate goal attainment was further attenuated and no longer significant at two years (ORunadj 1.07; 95% CI 0.75, 1.51) (Table 2). Absolute differences in urate concentration (−1.62 ± 1.85 vs. −1.41 ± 2.02 mg/dl; p = 0.06), final level achieved (6.8 ± 1.7 vs. 7.0 ± 1.8; p=0.02) and proportion of days covered (0.68 ± 0.29 vs. 0.61 ± 0.29; p<0.001) at one year favored the intervention (Table 3).
Table 3:
Changes in serum urate and allopurinol dosing from the time of index prescription to last follow-up
| Variable | Usual Care (n = 782) | Intervention (n = 681) | P-value |
|---|---|---|---|
| Serum Urate | |||
| Final serum urate, mean (SD), mg/dla | 7.0 (1.8) | 6.8 (1.7) | 0.02 |
| Serum urate change, mean (SD), mg/dla | −1.4 (2.0) | −1.6 (1.9) | 0.06 |
| Allopurinol Dosing | |||
| PDC during year 1, mean (SD)b | 0.61 (0.29) | 0.68 (0.29) | <0.001 |
| Final allopurinol dose, mean(SD), mg/dl | 211 (106) | 237 (107) | <0.001 |
| Final allopurinol dose, mg/day, % | |||
| <100 | 0.5 | 0.6 | <0.001 |
| 100 to <200 | 38.6 | 26.6 | |
| 200 to <300 | 18.3 | 21.4 | |
| 300 | 39.3 | 44.9 | |
| >300 | 3.3 | 6.5 | |
| Allopurinol dose change, % | <0.001 | ||
| No change | 79.3 | 63.4 | |
| Increase | 18.0 | 33.0 | |
| Decrease | 2.7 | 3.5 |
No follow-up serum urate available for 117 (15%) usual care patients and 83 (12%) intervention patients; either baseline or follow-up (needed to calculate change) were missing in 239 (31%) usual care patients and 211 (31%) intervention patients; mean (SD) interval between laboratory measures was significantly shorter among intervention patients (249 ± 263 vs. 372 ± 274 days; p<0.001)
PDC = proportion of days covered
Group differences in allopurinol dosing during follow-up are shown in Table 3. Although baseline doses were similar, intervention patients were nearly twice as likely to receive allopurinol dose escalation (33% vs. 18%, p<0.001) and achieved a higher ending dose (237 vs. 211 mg/day; p<0.001). Likewise, intervention patients were more likely than usual care patients to achieve daily doses of ≥300 mg (51% vs. 43%, p<0.001) while dose decreases were uncommon (<4%) in both groups.
Medically attended gout flares occurring during six-month intervals of follow-up are shown in Figure 2. Not achieving statistical significance, flare rates were numerically higher among intervention patients during the first year of therapy and were lower during the second year. Two patients were diagnosed with severe cutaneous reactions during follow-up, one receiving usual care and an allopurinol dose of 100 mg/day (442 days post-prescription) and one in the intervention arm receiving a dose of 100 mg/day (369 days post-prescription).
Figure 2: Gout flare rate during the intervention period (year one) and follow-up post-intervention (year two).
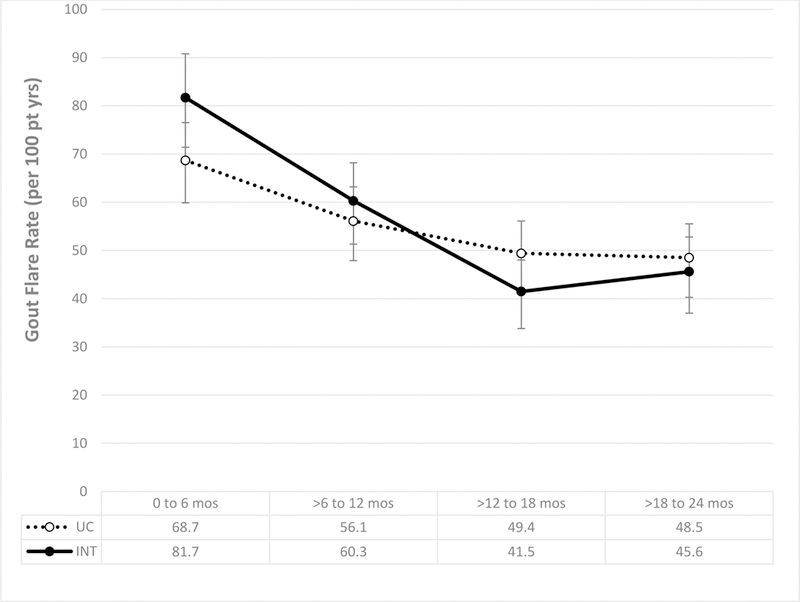
Gout flare rates (per 100 pt yrs) and 95% confidence intervals were calculated for each 6-month interval over a two-year follow-up period. Flare rates shown for gout patients receiving the intervention (solid line) and usual care (dotted line).
Discussion
Described as potentially “curative”25,26, urate-lowering is a key tenet of effective gout management. Despite the availability of efficacious and well-tolerated agents, gout management and related outcomes remain suboptimal. This deficiency has been attributed not only to poor patient adherence but also to inadequate prescribing practices27. Although subspecialty management guidelines support gradual dose titration of urate-lowering agents to achieve a target urate goal14,28, this approach is rarely implemented in real-world practice. Rather, providers more often administer allopurinol in fixed doses without escalation, infrequently prescribing doses in excess of 300 mg/day10,19. Results from randomized trials and observational studies have consistently demonstrated fixed-dosing strategies to be insufficient for the vast majority of gout sufferers10,19,29,30. In this large site-randomized study, we examined the implementation of a highly scalable pharmacist-led intervention as a means of overcoming the clinical inertia that characterizes urate-lowering, with the ultimate goal of improving outcomes.
Our relatively “light touch” intervention led to an approximate 70% improvement in allopurinol adherence and a more than 2-fold higher proportion of patients achieving a target urate goal after one year. Likewise, those receiving the intervention were more than twice as likely to receive allopurinol dose escalation and achieved a higher ending dose. The benefits observed with adherence and urate goal attainment during the first year, however, were attenuated after two years of follow-up suggesting limited durability and the potential need of continued patient engagement to sustain benefit. Furthermore, after limiting analyses to treatment adherent patients, group differences in urate goal attainment were attenuated at one-year and non-significant after two years, suggesting that the impact of this intervention on the achievement of optimal outcomes was, in part, driven by its promotion of compliance.
The intervention’s impact was clearly limited as a majority of gout patients exposed to the intervention never received dose increases, most failed to achieve target SU goal, and only half were adherent. Modest effects on adherence may be particularly noteworthy given the role of allopurinol adherence in the achievement of optimal disease outcomes31. Results from our study contrast with those from a far more intensive one-year single center intervention that was co-administered by a specialty nurse and rheumatologist and incorporated patient education with an individualized gout management plan32. In this study of 106 gout patients administered urate-lowering treatment, 96 (91%) completed one-year follow-up with the vast majority (92%) achieving urate goal. In a five-year follow-up, 68 of 75 (91%) participants responding to a mailed questionnaire reported treatment persistence and most reported being adherent33. Of these, 65 participated in a follow-up visit and most (86%) were at urate goal five years after treatment initiation. Although this prior study lacked a control group and relied on self-reports of adherence, these results suggest that a more intensive intervention - one that integrates patient education with regular in-person patient-provider interactions - could lead to greater and more durable improvements in adherence and outcomes. The potential importance of integrating patient education is further highlighted in reports demonstrating that gout-specific knowledge deficits (common among gout sufferers) might be amenable to relatively simple educational interventions delivered in part or wholly by non-physician providers such as pharmacists34,35. It is possible that a more resource-intensive strategy such as that described by Rees et al could be deployed in a stepwise, escalating fashion only after the failure of a less intensive (and more scalable) intervention (such as the one described in this study, delivered to 681 patients by a single half-time pharmacist).
In a 26-week study of 77 gout patients, “virtual” pharmacy-based management led to significant improvements in urate goal attainment36. Although improvements reported with the intervention were similar in magnitude to those observed in our study (35% obtaining urate goal at 26 weeks vs. 30% at one year), there were important differences. In addition to its smaller sample size and shorter follow-up, not all usual care patients in the former study received urate-lowering therapy whereas allopurinol receipt was universal in our study. Moreover, details specific to the treatment used, dosing, and dose escalation were not provided in the previous report, thus it is unclear how the virtual intervention impacted these potentially important mediating factors. Differences also existed between usual care groups examined as reflected in mean urate changes observed over follow-up, −1.4 mg/dl in our study compared to an increase of 0.2 mg/dl in the prior study36.
Although our study included interventions incorporating a treat-to-target paradigm, the merit of this strategy has recently come under scrutiny with some advocating a “treat-to-avoid symptoms” approach that emphasizes the importance of reducing or eliminating gout flares37. Although differences did not reach statistical significance, our intervention led to a numerically lower flare rate during the second year of observation. These results follow those from prior studies demonstrating that flare rates, after an initial increase following treatment initiation, dissipate and are nearly abolished over time with maintenance of urate levels <6.0 mg/dl30,38. Recognizing that allopurinol dose escalation is associated with an increased risk of severe cutaneous reactions, we examined the frequency of this complication. In line with reported frequencies 39,40, we observed two related events, one with usual care and one with the intervention. Both cases occurred with “low dose” allopurinol (100 mg/day) more than one year after allopurinol initiation, outside the risk window typically attributed to hypersensitivity41.
There are strengths to this study, including its large study population, site randomization, systematic approach to patient inclusion, real-world setting, and available links (for the study pharmacist) to detailed administrative and pharmacy-dispensing data in near real-time. Limitations include the use of passive follow-up to define flares, recognizing that this likely misses less severe flares. Likewise, our use of passive follow-up to identify adverse effects prohibited analyses of less severe events. We also recognize that additional technology-based contacts (e.g. via Smartphones or email messages) might have augmented the intervention, although these were not technically feasible in this study. Finally, given the reliance on administrative datasets, measures of gout severity and other disease characteristics were not available. Although this information would inform generalizability, similarities between our study participants with other gout populations (e.g. predominantly male, older, marked hyperuricemia in the absence of treatment, frequent comorbidity) add support to the external validity of these findings.
In summary, a relatively simple intervention leveraging pharmacists and automated telephone technology led to significant improvements in adherence and achievement of urate goal in gout patients initiating allopurinol. However, a significant proportion of gout patients were refractory to the intervention. Thus, while this light-touch, low-tech intervention was effective for some patients, additional efforts will be needed to ensure optimal management for an even greater proportion of gout patients.
Clinical Significance.
A multi-faceted pharmacist-led intervention incorporating automated telephone technology resulted in significant improvements in gout care.
Gout patients receiving this intervention were more adherent to therapy and more likely to achieve serum urate goals that have been associated with improved outcomes.
Although efficacious, a significant proportion of patients were refractory to the intervention, suggesting that additional patient engagement will be needed to achieve better outcomes for larger proportions of patients.
Acknowledgments
FUNDING SOURCE: This work is funded by the National Institutes of Health (NIH) / National Institute of Arthritis and Musculoskeletal and Skin Diseases (P50AR060772). Dr. Mikuls receives research support from NIH/National Institute of General Medical Sciences (U54GM115458) and the National Institute on Alcohol Abuse and Alcoholism (R25AA020818).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
TRIAL REGISTRATION: NCT 02790463 https://www.ClinicalTrials.gov
CONFLICT OF INTEREST: The authors have no conflict of interest to declare.
References
- 1.Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum 2011;63(10):3136–3141. [DOI] [PubMed] [Google Scholar]
- 2.Choi HK, Curhan G. Independent impact of gout on mortality and risk for coronary heart disease. Circulation 2007;116(8):894–900. [DOI] [PubMed] [Google Scholar]
- 3.Garg R, Sayles HR, Yu F, et al. Gout-related health care utilization in US emergency departments, 2006 through 2008. Arthritis Care Res 2013;65(4):571–577. [DOI] [PubMed] [Google Scholar]
- 4.Kleinman NL, Brook RA, Patel PA, et al. The impact of gout on work absence and productivity. Value Health 2007;10(4):231–237. [DOI] [PubMed] [Google Scholar]
- 5.Singh JA, Strand V. Gout is associated with more comorbidities, poorer health-related quality of life and higher healthcare utilisation in US veterans. Ann Rheum Dis 2008;67(9):1310–1316. [DOI] [PubMed] [Google Scholar]
- 6.Mikuls TR. Urate-lowering therapy. In: Firestein GS, Budd RC, Gabriel SB, McInnes IB, O’Dell JR, eds. Kelley & Firestein’s Textbook of Rheumatology Vol I 10th ed. Philadelphia: Elsevier; 2017:1061–1074. [Google Scholar]
- 7.Agematsu K, Nagumo H, Yang FC, et al. B cell subpopulations separated by CD27 and crucial collaboration of CD27+ B cells and helper T cells in immunoglobulin production. Eur J Immunol 1997;27(8):2073–2079. [DOI] [PubMed] [Google Scholar]
- 8.Briesacher BA, Andrade SE, Fouayzi H, Chan KA. Comparison of drug adherence rates among patients with seven different medical conditions. Pharmacotherapy 2008;28(4):437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cottrell E, Crabtree V, Edwards JJ, Roddy E. Improvement in the management of gout is vital and overdue: an audit from a UK primary care medical practice. BMC Fam Pract 2013;14:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rashid N, Coburn BW, Wu YL, et al. Modifiable factors associated with allopurinol adherence and outcomes among patients with gout in an integrated healthcare system. J Rheumatol 2015;42(3):504–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roddy E, Zhang W, Doherty M. Concordance of the management of chronic gout in a UK primary-care population with the EULAR gout recommendations. Ann Rheum Dis 2007;66(10):1311–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh JA, Hodges JS, Asch SM. Opportunities for improving medication use and monitoring in gout. Ann Rheum Dis 2009;68(8):1265–1270. [DOI] [PubMed] [Google Scholar]
- 13.Solomon DH, Avorn J, Levin R, Brookhart MA. Uric acid lowering therapy: prescribing patterns in a large cohort of older adults. Ann Rheum Dis 2008;67(5):609–613. [DOI] [PubMed] [Google Scholar]
- 14.Khanna D, Fitzgerald JD, Khanna PP, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken) 2012;64(10):1431–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richette P, Doherty M, Pascual E, et al. 2016 updated EULAR evidence-based recommendations for the management of gout. Ann Rheum Dis 2017;76(1):29–42. [DOI] [PubMed] [Google Scholar]
- 16.Wortmann RL, Macdonald PA, Hunt B, Jackson RL. Effect of prophylaxis on gout flares after the initiation of urate-lowering therapy: analysis of data from three phase III trials. Clin Ther 2010;32(14):2386–2397. [DOI] [PubMed] [Google Scholar]
- 17.Dalbeth N, House ME, Horne A, Petrie KJ, McQueen FM, Taylor WJ. Prescription and dosing of urate-lowering therapy, rather than patient behaviours, are the key modifiable factors associated with targeting serum urate in gout. BMC Musculoskelet Disord 2012;13:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stamp LK, Merriman TR, Barclay ML, et al. Impaired response or insufficient dosage? Examining the potential causes of “inadequate response” to allopurinol in the treatment of gout. Semin Arthritis Rheum 2014;44(2):170–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coburn BW, Michaud K, Bergman DA, Mikuls TR. Allopurinol dose escalation and mortality among patients with gout: a national propensity-matched cohort study. Arthritis Rheumatol 2018. [DOI] [PubMed]
- 20.Coburn BW, Cheetham TC, Rashid N, et al. Rationale and design of the randomized evaluation of an ambulatory care pharmacist-led intervention to optimize urate lowering pathways (ramp-up) study. Contemporary clinical trials 2016;50:106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koebnick C, Langer-Gould AM, Gould MK, et al. Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J 2012;16(3):37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oake N, Jennings A, van Walraven C, Forster AJ. Interactive voice response systems for improving delivery of ambulatory care. Am J Manag Care 2009;15(6):383–391. [PubMed] [Google Scholar]
- 23.Nau DP. Proportion of days covered (pdc) as a preferred method of measuring medication adherence http://pqaalliance.org/resources/adherence.asp. Accessed April 25, 2018.
- 24.Halpern R, Fuldeore MJ, Mody RR, Patel PA, Mikuls TR. The effect of serum urate on gout flares and their associated costs: an administrative claims analysis. J Clin Rheumatol 2009;15(1):3–7. [DOI] [PubMed] [Google Scholar]
- 25.Doherty M, Jansen TL, Nuki G, et al. Gout: why is this curable disease so seldom cured? Ann Rheum Dis 2012;71(11):1765–1770. [DOI] [PubMed] [Google Scholar]
- 26.Perez-Ruiz F. Treating to target: a strategy to cure gout. Rheumatology (Oxford) 2009;48 Suppl 2:ii9–ii14. [DOI] [PubMed] [Google Scholar]
- 27.Coburn BW, Mikuls TR. The problem with gout is that it’s still such a problem. J Rheumatol 2016;43(8):1453–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang W, Doherty M, Bardin T, et al. EULAR evidence based recommendations for gout. Part II: Management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis 2006;65(10):1312–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Becker MA, Schumacher HR Jr., Wortmann RL, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med 2005;353(23):2450–2461. [DOI] [PubMed] [Google Scholar]
- 30.Schumacher HR Jr., Becker MA, Wortmann RL, et al. Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: a 28-week, phase III, randomized, double-blind, parallel-group trial. Arthritis Rheum 2008;59(11):1540–1548. [DOI] [PubMed] [Google Scholar]
- 31.Coburn BW, Bendlin KA, Sayles H, Meza J, Russell CL, Mikuls TR. Allopurinol medication adherence as a mediator of optimal outcomes in gout management. J Clin Rheumatol 2017;23(6):317–323. [DOI] [PubMed] [Google Scholar]
- 32.Rees F, Jenkins W, Doherty M. Patients with gout adhere to curative treatment if informed appropriately: proof-of-concept observational study. Ann Rheum Dis 2013;72(6):826–830. [DOI] [PubMed] [Google Scholar]
- 33.Abhishek A, Jenkins W, La-Crette J, Fernandes G, Doherty M. Long-term persistence and adherence on urate-lowering treatment can be maintained in primary care-5-year follow-up of a proof-of-concept study. Rheumatology (Oxford) 2017;56(4):529–533. [DOI] [PubMed] [Google Scholar]
- 34.Counsell AB, Nguyen AD, Baysari MT, Kannangara DRW, McLachlan AJ, Day RO. Exploring current and potential roles of Australian community pharmacists in gout management: a qualitative study. BMC Fam Pract 2018;19(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fields TR, Rifaat A, Yee AMF, et al. Pilot study of a multidisciplinary gout patient education and monitoring program. Semin Arthritis Rheum 2017;46(5):601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldfien R, Pressman A, Jacobson A, Ng M, Avins A. A pharmacist-staffed, virtual gout management clinic for achieving target serum uric acid levels: a randomized clinical trial. Perm J 2016;20(3):18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qaseem A, Harris RP, Forciea MA. Clinical Guidelines Committee of the American College of P. Management of Acute and Recurrent Gout: A Clinical Practice Guideline From the American College of Physicians. Ann Intern Med 2017;166(1):58–68. [DOI] [PubMed] [Google Scholar]
- 38.Schumacher HR Jr., Becker MA, Lloyd E, MacDonald PA, Lademacher C. Febuxostat in the treatment of gout: 5-yr findings of the FOCUS efficacy and safety study. Rheumatology (Oxford) 2009;48(2):188–194. [DOI] [PubMed] [Google Scholar]
- 39.Kim SC, Newcomb C, Margolis D, Roy J, Hennessy S. Severe cutaneous reactions requiring hospitalization in allopurinol initiators: a population-based cohort study. Arthritis Care Res (Hoboken) 2013;65(4):578–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang CY, Chen CH, Deng ST, et al. Allopurinol use and risk of fatal hypersensitivity reactions: a nationwide population-based study in taiwan. JAMA Intern Med 2015;175(9):1550–1557. [DOI] [PubMed] [Google Scholar]
- 41.Stamp LK, Taylor WJ, Jones PB, et al. Starting dose is a risk factor for allopurinol hypersensitivity syndrome: a proposed safe starting dose of allopurinol. Arthritis Rheum 2012;64(8):2529–2536. [DOI] [PubMed] [Google Scholar]


