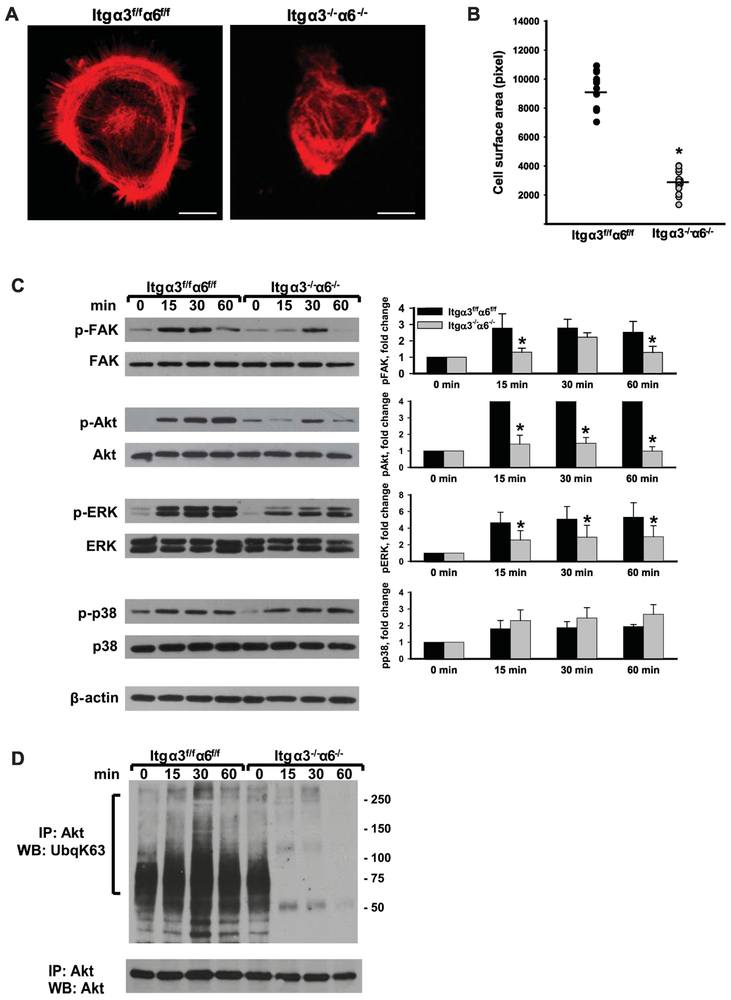
Figure 2. Integrin subunits α3 and α6 regulate CD cell spreading on LM-511 as well as Akt phosphorylation and K63-linked polyubiquitination.
(A-B) Spreading of Itgα3f/fα6f/f and Itgα3−/−α6−/− CD cells on LM-511 was evaluated 1 h after plating. (A) Representative confocal images of cells stained with rhodamine–phalloidin are shown; bar: 10 μM. (B) The individual values of cell surface area (in pixels) of 15-30 cells with the mean is shown; *p≤0.05 between Itgα3f/fα6f/f and Itgα3−/−α6f/f CD cells. (C-D) Itgα3f/fα6f/f and Itgα3−/−α6−/− CD cells were plated on LM-511 (0.5 μg/ml) and lysed 15, 30 and 60 min later. Cell lysates were subjected to immunoblot analysis for phosphorylation of FAK, Akt, p38, ERK1/2 (C), or to immunoprecipitation with an anti-Akt antibody followed by immunoblot analyses for K63-linked polyubiquitination and Akt (D). Time point “0” represents non-adherent cells. Levels of phosphorylated proteins were measured by densitometry, normalized to total protein and β-actin levels, and expressed as fold-change relative to cells left in suspension, “0” time point. Values are the mean and ±SEM of 3 independent experiments; *p≤0.05 between Itgα3f/fα6f/f and Itgα3−/−α6f/f CD cells of the corresponding time point.