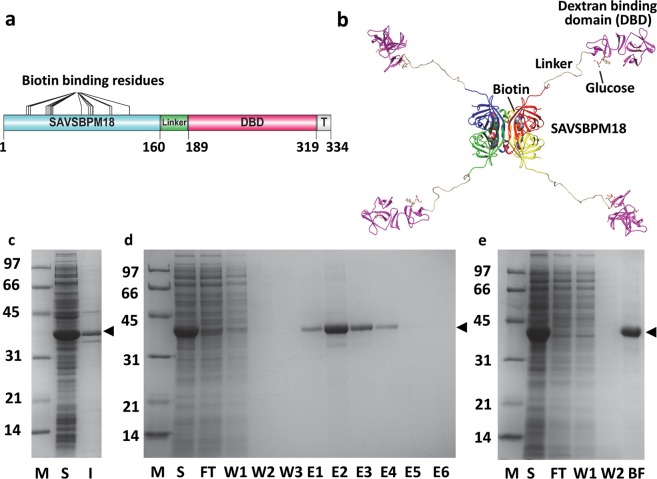
Figure 1.
Primary and modelled structures of SAVSBPM18-Linker-Dextran-binding domain (M18-L-DBD), and its purification and immobilization to Sephadex. (a) Organization of various domains [engineered streptavidin SAVSBPM18, linker, dextran-binding domain (DBD) and a short C-terminal tail sequence (T)] in M18-L-DBD. Amino acid residues that mark the boundary of the domains are listed. (b) A modelled structure of M18-L-DBD. The four subunits in SAVSBPM18 are colored in red, yellow, green and blue, respectively. DBD is colored in purple. (c) SDS-PAGE showing intracellular production of M18-L-DBD in E. coli BL21[pET29B-M18-L-DBD]. (d) Purification of M18-L-DBD using biotin-agarose. Fractions were analyzed by SDS-PAGE. (e) SDS-PAGE analysis of the binding of M18-L-DBD to Sephadex G-100. All samples in panels c–e were boiled before loading. Arrowhead indicates the position of M18-L-DBD. M, molecular weight markers (sizes in kDa); S, intracellular soluble fraction; I, intracellular insoluble fraction; FT, flow-through fraction; W, wash fractions; E, elution fractions; BF, bound fraction. Gel profiles shown in panels c, d and e are from different gels.