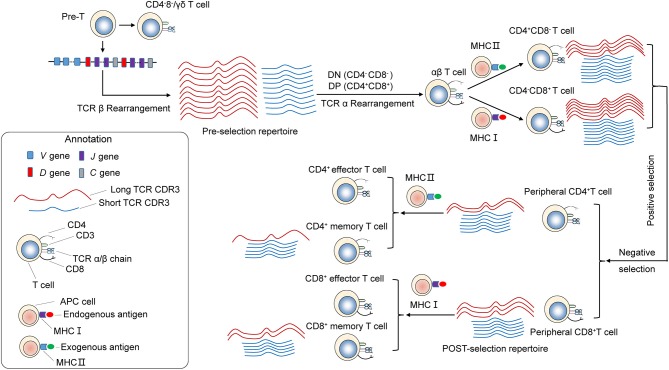
Figure 7.
Skewing of the TCR repertoire during thymic selection and antigen-driven selection. T cells differentiate and mature through several thymocyte developmental stages. T-cell precursors differentiate into CD4−CD8− [double-negative (DN)]. TCRβ gene locus rearrangement and expression first occur at the DN3 stage. A random combination of VDJ genes undergoes rearrangement, yielding a Pre-selection repertoire of short and long TCRB CDR3s. Subsequently, it becomes CD4+CD8+ double-positive (DP) cortical thymocytes. TCRα gene locus rearrangement take place in DP stage. As a result, functional TCRα/β heterodimer is expressed on the surface. These DP cells through a highly ordered developmental process called positive selection, during which engagement of TCR on DP cells by self-peptide MHC complex (pMHC) from thymic epithelial cells with appropriate avidity will deliver signals to DP cells and enable these cells to proceed into MHCII restricted CD4+ single positive or MHCI restricted CD8+ single positive thymocytes. Interaction with pMHCI may need longer TCRβ CDR3 than that of pMHCII. Therefore, a significant increase in average CDR3 size is observed in CD8+ T cells compared with CD4+ T cell. Then, T cells with excessive self reactivity are deleted in the thymus, a process called negative selection. Through the above process and selection, generating the Post-selection repertoire with an increased frequency of shorter TCRβ CDR3s, which undergo enrichment during thymic selection. The peripheral naive repertoire is shaped by antigen encounter. MHC Class I proteins are primarily involved with the presentation of intracellular antigen to cytotoxic CD8+ T cells, while MHC class II proteins present extracellular antigen to CD4+ T helper cells. It may be that short TCRβ CDR3s can be recognition of most antigen presented by MHC, and in result short CDR3s were further enriched in memory T cell repertoire.