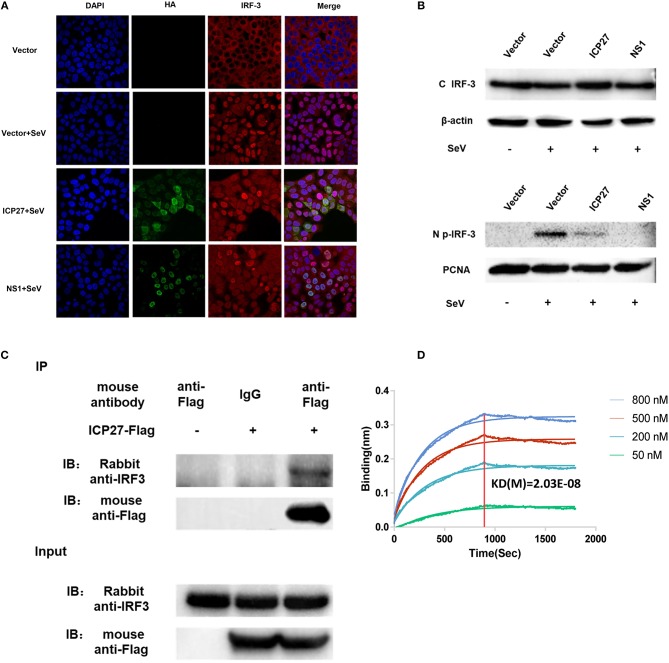
Figure 5.
HSV-2 ICP27 blocks IRF3 activation by physical interaction. (A) HSV-2 ICP27 interferes with the nuclear translocation of IRF3. HeLa cells were seeded in 35 mm glass-bottom dishes and transfected with empty vector pcDNA3.1(+), plasmid expressing HSV-2 ICP27-HA or influenza virus NS1-HA. At 24 h post-transfection, cells were stimulated with or without 100 HAU ml−1 SeV for 16 h and prepared for immunofluorescence assay. IRF3 was detected under 647 nm wavelength, ICP27-HA or NS1-HA under 488 nm wavelength, and nuclei under 405 nm wavelength. (B) HSV-2 ICP27 inhibits IRF3 phosphorylation. HEK 293T cells were seeded in 6-well plates and transfected with pcDNA3.1(+), plasmid expressing HSV-2 ICP27 or influenza virus NS1. At 24 h post-transfection, cells were stimulated with or without 100 HAU ml−1 SeV for 16 h. Nuclear and cytoplasmic proteins were isolated, and phosphorylated IRF3 was detected with a p-IRF3 mAb. (C) HSV-2 ICP27 interacts with endogenous IRF3. HEK 293T cells were seeded in 6-well plates and transfected with pcDNA3.1(+) or HSV-2 ICP27-Flag. At 24 h post-transfection, cells were stimulated with or without 100 HAU ml−1 SeV for 16 h and lysed for immunoprecipitation assay. One representative experiment out of three is shown (A–C). (D) Kinetics of ICP27-IRF3 binding. The kinetics of binding was performed on a Forte-Bio Octet Red System. Purified IRF3 was conjugated with biotin at a molecular weight ratio of 1:3. Ten microgram/milliliter biotinylated IRF3-Flag was coupled to Biosenors and immersed in different concentration of ICP27-Flag (50, 200, 500, or 800 nM) for association and disassociation. The response in nm shift was recorded as a function of time. KD (M) = 2.03E-08.