Figure 2. SPPL2c cleaves selected tail‐anchored (TA) proteins with a substrate spectrum distinct from SPP.
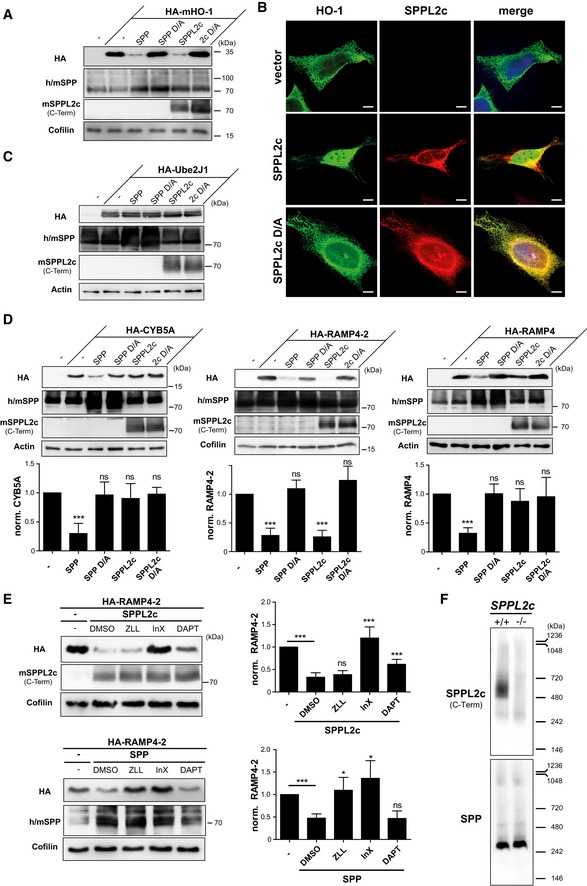
- HEK293 cells were transiently transfected with murine N‐terminally HA‐tagged heme oxygenase 1 (HA‐mHO‐1) alone or in combination with active or inactive (D/A) murine SPP or SPPL2c. Substrate levels were determined by Western blot analysis with anti‐HA. For detection of SPP, an antiserum recognising both endogenous human (h) and overexpressed murine (m) SPP was employed. Murine SPPL2c was visualised using the antiserum generated against the C‐terminus of the protein. Actin was detected to control for equal protein loading.
- Immunofluorescence analysis of HeLa cells transiently expressing murine HO‐1 (HA‐mHO‐1) alone or together with active or inactive (D/A) SPPL2c‐myc. Substrate and proteases were visualised using the HA and Myc epitopes, respectively. Scale bars, 10 μm.
- The TA protein Ube2J1 is not proteolysed by co‐expressed SPP or SPPL2c in HEK293 cells. Western blot analysis was performed as in (A).
- Differential cleavage of CYB5a, RAMP4‐2 and RAMP4 by SPPL2c and SPP. Substrates and proteases were co‐expressed in HEK293 cells followed by Western blot analysis as described above. Densitometric quantification from at least n = 3 independent experiments was performed. Substrate levels were normalised and compared to cells without protease overexpression. Mean ± SD; unpaired Student's t‐test; ***P ≤ 0.001; ns, non‐significant.
- HEK293 cells expressing HA‐RAMP4‐2 alone or in combination with SPPL2c or SPP were treated with 100 μM (Z‐LL)2‐ketone (ZLL), 5 μM inhibitor X (InX), 5 μM DAPT or DMSO as control. RAMP4‐2 band intensity was quantified from blots of three independent experiments and normalised to cells just overexpressing the substrate. Mean ± SD, unpaired Student's t‐test; ***P ≤ 0.001, *P ≤ 0.05; ns, non‐significant.
- Microsomes isolated from testis of wild‐type and SPPL2c −/− mice were solubilised in 1% digitonin, and proteins were separated by blue‐native PAGE. After transfer to PVDF membrane, SPPL2c and SPP were detected using the polyclonal antisera introduced above.
Source data are available online for this figure.
