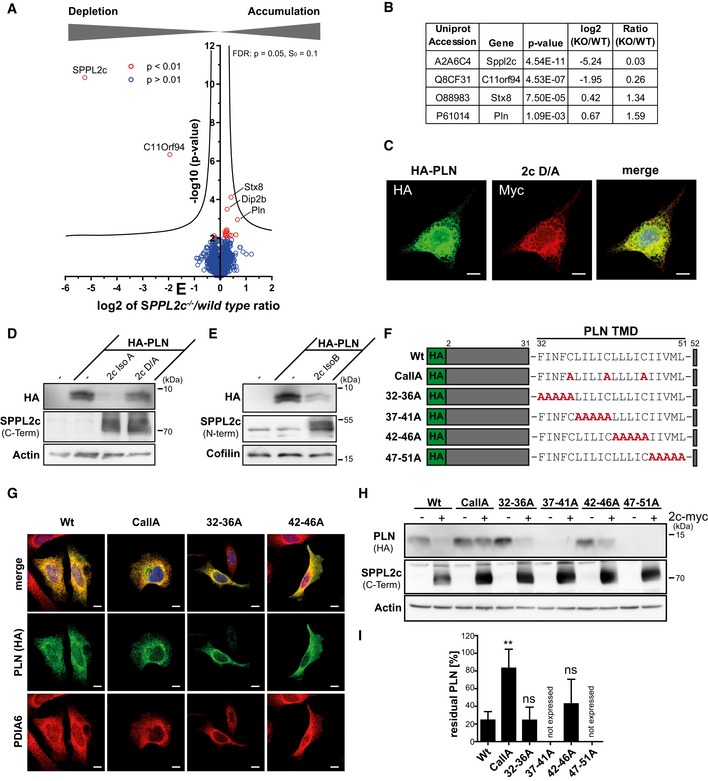
Figure 4. Identification of phospholamban (PLN) as novel substrate of SPPL2c.
-
ACarbonate‐washed total membrane fractions from wild‐type and SPPL2c −/− testis (n = 5) were subjected to a label‐free quantitative proteome analysis. For each quantified protein, a log2 intensity ratio between SPPL2c‐deficient and wild‐type samples was calculated. Negative values indicate a depletion and positive values an enrichment in SPPL2c −/− samples. In the depicted volcano plot, the negative log10 of the P value (y axis) is plotted versus the log2 intensity ratio of SPPL2c −/− versus wild‐type samples (x axis). All proteins above the significance level of P < 0.01 (unpaired Student's t‐test) are coloured in red. The hyperbolic curve indicates the threshold for a permutation‐based FDR correction for multiple hypotheses with P = 0.05 and s0 = 0.1.
-
BQuantification of SPPL2c, C11orf94, Syntaxin 8 (Stx8) and Phospholamban (PLN) in the proteomic dataset. A two‐sided Student's t‐test was performed.
-
CHeLa cells were transiently transfected with N‐terminally HA‐tagged PLN (HA‐PLN) and inactive (D/A) SPPL2c‐myc. The expressed proteins were visualised by indirect immunofluorescence via their HA and Myc epitopes. Scale bars, 10 μm.
-
D, EHEK293 cells were transiently transfected with HA‐PLN alone or in combination with active or inactive (D/A) murine SPPL2c isoform A (D) or isoform B (E). Substrate processing was analysed by Western blotting with anti‐HA. SPPL2c expression was confirmed with the antiserum against the C‐terminal epitope. Actin was detected to confirm equal protein loading.
-
FScheme of mutants generated for analysis of determinants within the PLN transmembrane domain (TMD) required for SPPL2c‐mediated intramembrane proteolysis.
-
GSubcellular localisation of the PLN mutants was analysed by indirect immunofluorescence using anti‐HA in combination with anti‐protein disulphide isomerase A6 (PDIA6) to stain the ER. The 37–41A and 47–51 PLN mutants were not expressed in relevant amounts. Scale bars, 10 μm.
-
HHEK293 cells were transiently transfected with wild type or mutated HA‐PLN alone or together with SPPL2c isoform A.
-
IQuantification of n = 5 experiments as shown in (H). For each PLN variant, the residual PLN in SPPL2c co‐expressing cells is depicted as % of PLN in the absence of the protease. Mean ± SD; one‐way ANOVA with Dunnett's post hoc test. **P ≤ 0.01, ns = not significant.
Source data are available online for this figure.
