-
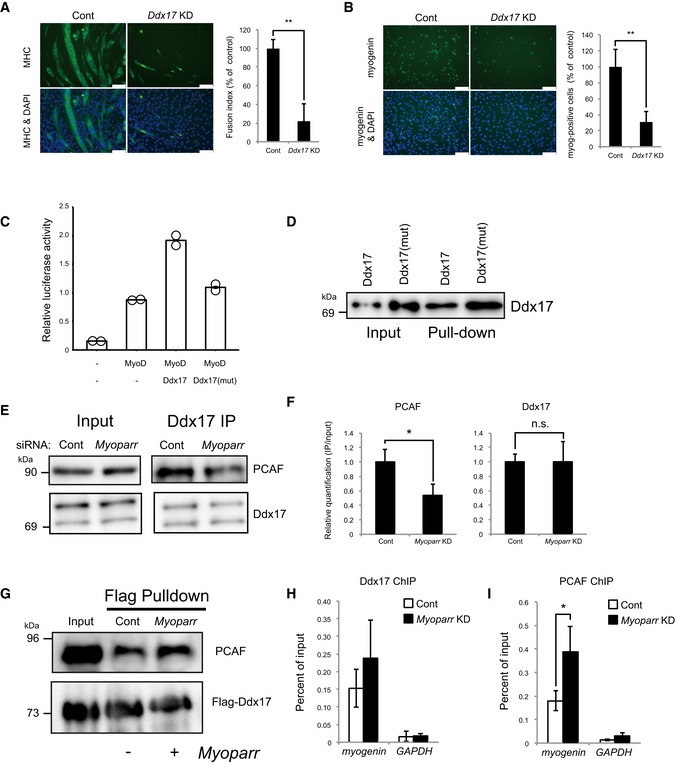
A
Significantly decreased MHC expression in Ddx17‐depleted C2C12 cells is shown by immunocytochemistry. Nuclei were counterstained with DAPI. Scale bar, 100 μm. Fusion index is shown as percent of the control. n = 3, mean ± SD. **P < 0.01 (unpaired two‐tailed Student's t‐test).
-
B
Immunocytochemistry for myogenin in Ddx17‐depleted C2C12 cells. Scale bar, 100 μm. Myogenin‐positive cells are shown as percent of the control. n = 4, mean ± SD. **P < 0.01 (unpaired two‐tailed Student's t‐test).
-
C
Relative luciferase activities of the ‐1650‐Luc by the combination of MyoD and Ddx17 or Ddx17 mutant (K142R). Bars indicate the average of two independent experiments, and open circles represent the values of each experiment.
-
D
Ddx17 or Ddx17 mutant (K142R) was pulled down by full‐length Myoparr and then detected by Western blot.
-
E
Reduced interaction between endogenous Ddx17 and PCAF in Myoparr‐depleted C2C12 cells. After Myoparr KD, the cell lysates were subjected to immunoprecipitation (IP) with a Ddx17‐specific antibody 36 h after differentiation induction (right panel). Each lysate was loaded as an input (left panel).
-
F
Relative quantification of (E) is shown as IP/input ratio. n = 3, mean ± SD. *P < 0.05. n.s., not significant (unpaired two‐tailed Student's t‐test).
-
G
Increased interaction between Ddx17 and PCAF by Myoparr. PCAF protein was pulled down by 3xFlag‐Ddx17 in the presence or absence of Myoparr using a Flag antibody and then detected by Western blot.
-
H, I
ChIP‐qPCR detection of Ddx17 (H) and PCAF (I) occupancies at the myogenin locus in Myoparr‐depleted differentiating C2C12 cells. The data were normalized to input values. n = 3, mean ± SD. *P < 0.05 (unpaired two‐tailed Student's t‐test).