Table 2. Effect of the substituents on the ene-donor.
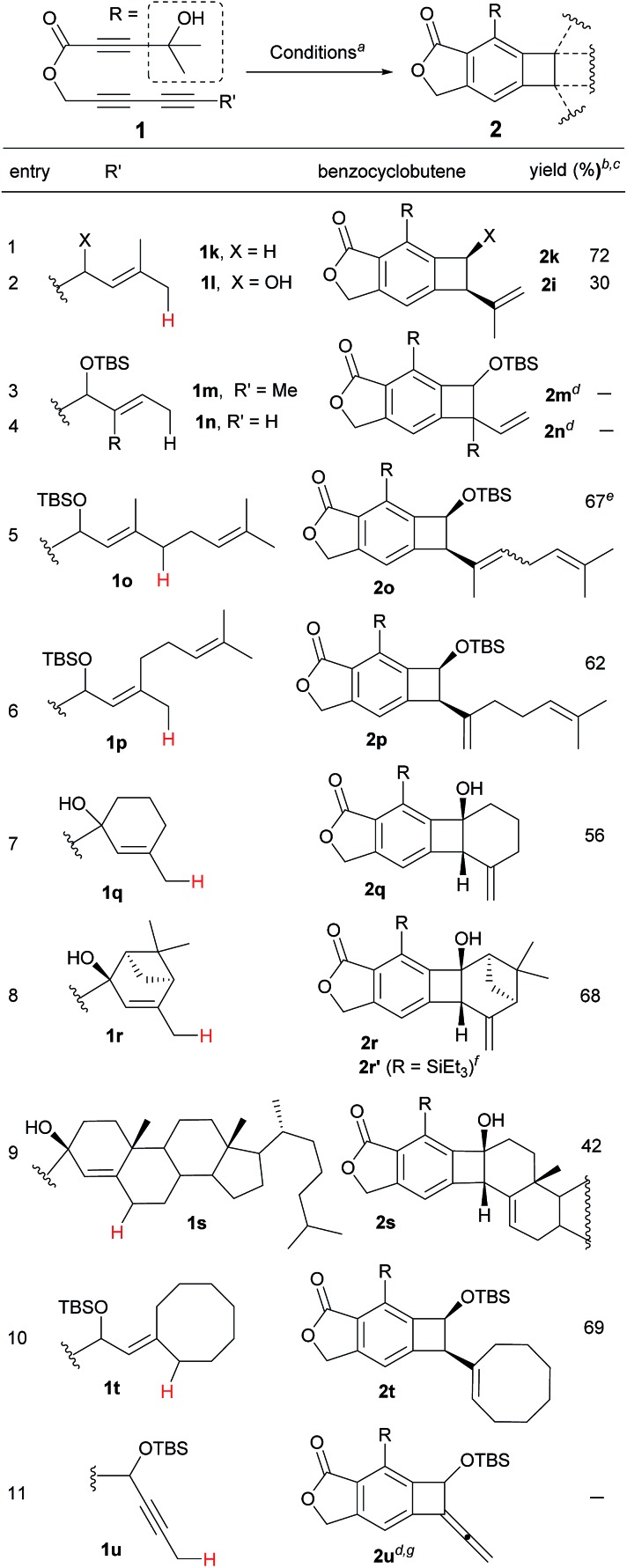
|
aConditions: toluene, 130 °C, 14 h.
bIsolated yield.
c cis-Diastereomer only.
dDecomposition or polymerization of the aryne intermediates.
e E/Z-mixture (2.5 : 1).
fWith R = SiEt3, 2r′ was obtained in 46% yield at 150 °C at 40 h.
gIntermolecular Alder-ene reaction with β-pinene (5 equiv.) of 1u provided 2u′ (see ref. 13).
