Abstract
Background:
The aim of this study was to assess the serum chitotriosidase (ChT) and neopterin levels in patients with ankylosing spondylitis (AS) and to evaluate whether serum ChT and neopterin levels are related to disease activity.
Methods:
A total of 86 patients with AS were included in the study. Patients were divided into two groups based on Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) scores: The active AS patients group included 40 patients who had a BASDAI score ⩾4. The inactive AS patients group included 46 patients who had a BASDAI score <4. We compared the serum level of erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), ChT and neopterin between the two groups.
Results:
Active AS patients had significantly higher ESR, CRP, serum ChT and neopterin levels compared with the inactive AS patients group (p < 0.05). Positive correlations were found between serum ChT levels and ESR (r = 0.87, p = 0.005), and CRP levels (r = 0.86, p = 0.006). Also, there was a positive significant correlation between serum ChT levels and BASDAI scores (r = 0.67, p = 0.03). No correlation was found between serum neopterin levels and the BASDAI scores, ESR, and CRP levels (p > 0.05). Higher disease activity (BASDAI score ⩾4) was found to be associated with ChT (p = 0.012) in the multiple logistic regression analysis.
Conclusion:
The present study emphasized that serum ChT levels can be useful in the determination of the disease activity of AS patients.
Keywords: ankylosing spondylitis, chitotriosidase, disease activity, neopterin
Introduction
Ankylosing spondylitis (AS) is a chronic inflammatory rheumatic disease that primarily affects the spine and sacroiliac joints, and macrophages play a major role in the inflammation.1 Human chitotriosidase (ChT) is a member of the chitinase family and has the capability to hydrolyze chitin.2 Macrophages are able to produce large amounts of ChT when activated.3,4 Neopterin is a low-molecular weight compound derived from guanosine triphosphate. Human monocytes and macrophages constitute the most important source of neopterin stimulated by interferon-γ.5 Therefore, the serum levels of neopterin and ChT have been considered as a marker of macrophage activation.
Increased levels of ChT have been observed in patients with Alzheimer’s disease, multiple sclerosis, thalassemia and atherosclerosis all of which comprise lysosomal storage diseases and macrophage activation.4,6–8 Increased serum ChT levels were also observed in patients with sarcoidosis9 and juvenile idiopathic arthritis.10 The result of these studies has showed that serum ChT can be used in monitoring disease activity, in following treatment efficacy and in investigating disease prognosis. Neopterin has been also observed as a biochemical marker of immune system activation in some diseases such as polycystic ovary syndrome,11 rheumatoid arthritis,12 autoimmune diseases such as insulin-dependent diabetes mellitus, systemic lupus erythematosus, multiple sclerosis, coeliac disease,13,14 Crohn’s disease and ulcerative colitis.15
To the best of our knowledge, no study has yet been performed for investigating the serum neopterin and ChT levels in patients with AS. The aim of this study was to assess the serum ChT and neopterin levels in patients with AS and to evaluate whether serum ChT and neopterin levels are related to disease activity.
Materials and methods
Study design and patients
This prospective designed study was conducted with 86 patients fulfilling the modified New York criteria16 for AS. The patients were recruited from the physical medicine and rehabilitation outpatient clinic. The same researcher examined the patients and carried out the measurements of disease activity. Disease activity was measured with a Bath Ankylosing Spondylitis Disease Activity Index (BASDAI).17 The BASDAI consists of six questions pertaining to the five major symptoms of AS: fatigue, spinal pain, joint pain/swelling, areas of localized tenderness and morning stiffness. Patients were asked to answer each item using a 0–10 cm visual analogue scale, on which the patients could indicate their assessment along a 10 cm line ranging from 0 (‘no complaint at all’) to 10 (‘the most severe complaint’). The possible BASDAI score is ranged from 0 to 10. Scores of 4 or greater suggest active disease (uncontrolled, disease activity) while scores less than 4 suggest inactive disease (controlled, disease activity). We also recorded patients’ age, sex, duration of disease and drugs used by the patients such as disease-modifying anti-rheumatic drugs (DMARDs), anti-tumor necrosis factor (TNF) drugs and nonsteroidal anti-inflammatory drugs (NSAIDs).
Patients were divided into two groups based on BASDAI scores. The active AS patients group included 40 patients who had BASDAI score ⩾4. The inactive AS patients group included 46 patients who had BASDAI score <4. We compared the serum level of erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), ChT and neopterin between the two groups. Patients were excluded if they had acute or chronic infection disease, other chronic inflammatory rheumatic disease, lysosomal storage diseases or metabolic diseases. The study protocol was approved by the Hospital Ethical Committee and each patient provided written informed consent.
Blood sampling and analysis
Blood samples of all the participants were collected to determine the serum level of acute-phase reactants, ESR or CRP, and serum neopterin and ChT levels. Plasma samples of the neopterin and ChT were stored at −80°C for the analysis. Serum ChT activity determination was performed as described by Hollak and colleagues2 with minor modifications. Serum ChT activity was measured using a Microfluor fluorimeter (Bio-Tek Instruments, Neufahrn, Germany; excitation, 355 nm; emission, 460 nm). Serum ChT activities were expressed as nanomoles of substrate hydrolyzed per milliliter per hour (nmol/ml/h). The normal levels for ChT was 6.2–27.0 nmol/ml/h. Serum neopterin concentration was measured with a high-performance liquid chromatography device (Agilent Technologies 1200 Series System, Santa Clara, CA, USA) using a fluorometry detector, as previously defined by Fuchs and colleagues.18 The calculated overall intra-assay and inter-assay coefficient of variation (CV) was 1.2% and 2.6% respectively. The normal levels for neopterin was 3.5–15.0 nmol/l. Serum ChT activities and serum neopterin concentration were measured by the same clinicians of the Department of Biochemistry. CRP levels were measured by the immunoturbidimetric method (Schiapparelli Biosystems, The Netherlands) and levels lower than 3.23 mg/l were accepted to be negative. The Westergreen method was used to measure ESR in which the range between 1 and 20 mm/h was accepted as normal.
Statistical analysis
The sample size of 86 patients was based upon a sample size calculation, with an anticipated mean difference of 14 and a standard deviation of 8 in ChT level, and with an anticipated mean difference of 10 and a standard deviation of 6 in neopterin level, between the two groups, allowing for a p value of 0.05 and a power of 0.95.
Statistical analysis was performed using SPSS version 15.0 (SPSS, Chicago, IL). Categorical variables are presented as a proportion and percentage. Continuous variables are presented as mean ± standard deviation. Patients’ sex and medications were compared using the Pearson Chi-square test between the two groups. The comparisons between the two groups were performed using a nonparametric test (Mann–Whitney U test). A Spearman rank correlation was used to examine the relationship between the parameters. Multiple logistic regression analysis was performed to identify the influence of multiple variables (age, sex, duration of disease, and ChT) on a dependent variable (presence of active disease, BASDAI score ⩾4). We used receiver operating characteristic (ROC) plot analysis to evaluate and compare the performance of ESR, CRP and serum ChT in assessing disease activity. ROC analysis is a nonparametric method used to quantify the accuracy of the prediction. The level of statistical significance was set at p < 0.05.
Results
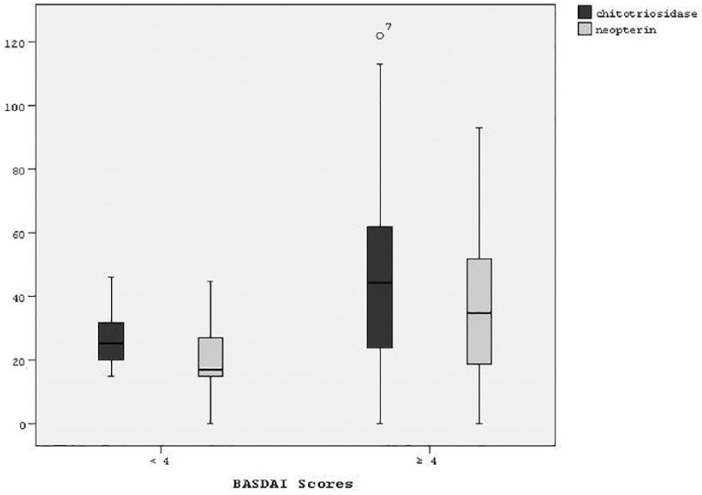
The distribution of clinical and biochemical parameters of the groups are shown in Table 1. There were no significant differences between the two groups in terms of age, sex, use of drug and duration of disease. The patients in the active disease group had significantly higher ESR, CRP, serum ChT and neopterin levels compared with patients in the inactive AS group (p < 0.05). The distribution of serum ChT and neopterin levels according to BASDAI scores are shown in Figure 1.
Table 1.
Comparison of clinical and biochemical parameters between the two groups.
| Characteristics | Active disease group (n = 40) | Inactive disease group (n = 46) | p |
|---|---|---|---|
| Age (mean ± SD); year | 37.4 ± 15.2 | 41.7 ± 15.0 | 0.11a |
| Sex | 26/20 | 0.54b | |
| Male | 20 (50%) | 26 (56.5%) | |
| Female | 20 (50%) | 20 (43.4%) | |
| Medication; number of patients | |||
| DMARD plus NSAID | 20 (50%) | 20 (43.4%) | 0.23b |
| NSAID alone | 7 (17.5%) | 9 (19.5%) | 0.67b |
| DMARD alone | 5 (12.5%) | 7 (15.2%) | 0.78b |
| Anti-TNF alone | 8 (20%) | 10 (21.7%) | 0.93b |
| Duration of disease (mean ± SD); month | 51.57 ± 33.43 | 46.21 ± 42.66 | 0.59a |
| ESR (mean ± SD); mm/h | 28.98 ± 23.00 | 8.14 ± 3.80 | 0.001 a |
| CRP (mean ± SD); mg/l | 15.16 ± 14.44 | 6.45 ± 5.94 | 0.03 a |
| ChT (mean ± SD); nmol/ml/h | 49.14 ± 31.40 | 26.50 ± 8.69 | 0.01 a |
| Neopterin (mean ± SD); nmol/l | 37.25 ± 25.36 | 20.71 ± 10.85 | 0.02 a |
As determined by the Mann–Whitney U test.
As determined by the Pearson Chi-square test.
Bold values indicate a p value <0.05.
ChT: chitotriosidase; CRP: C-reactive protein, DMARD, disease-modifying anti-rheumatic drug; ESR: erythrocyte sedimentation rate; NSAID, nonsteroidal anti-inflammatory drug; SD, standard deviation; TNF, tumor necrosis factor.
Figure 1.
The distribution of serum ChT and neopterin levels according to BASDAI scores.
BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; ChT, chitotriosidase.
When we investigated the serum level of ESR, CRP, neopterin and ChT among the patients in the active disease group, we found that 33% of the patients had a normal ESR level, 21% of the patients had a normal CRP level, 65% of the patients had a normal neopterin level, and 28% of the patients had a normal ChT level.
Positive correlations were found between serum ChT levels and ESR (r = 0.87, p = 0.005), and CRP levels (r = 0.86, p = 0.006). Also, there was positive significant correlation between serum ChT levels and BASDAI scores (r = 0.67, p = 0.03). No correlation was found between serum neopterin levels and the BASDAI scores, ESR, and CRP levels (p > 0.05). The results of correlation analyses were shown in Table 2.
Table 2.
Correlation analysis of serum ChT and neopterin levels with other parameters.
| Variables | Correlation with ChT |
Correlation with neopterin |
||
|---|---|---|---|---|
| r | p a | r | p a | |
| ESR | 0.87 | 0.005 | 0.36 | 0.18 |
| CRP | 0.86 | 0.006 | 0.54 | 0.23 |
| BASDAI scores | 0.67 | 0.03 | 0.25 | 0.42 |
Spearman correlation test was used.
Bold values indicate a p value <0.05.
BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; ChT: chitotriosidase; CRP, C-reactive protein; ESR: erythrocyte sedimentation rate;
Higher disease activity (BASDAI score ⩾4) was found associated with age (p = 0.043), ESR (p = 0.018) and ChT (p = 0.047) in the multiple logistic regression analysis (Table 3).
Table 3.
Multiple logistic regression analysis for disease activity in patients with ankylosing spondylitis (n = 86).
| Variables | Presence of active disease (BASDAI ⩾4) |
||
|---|---|---|---|
| Standardized ß coefficient | t | p | |
| Age | −0.56 | 0.946 | 0.056 |
| Sex | −0.746 | 0.474 | 0.383 |
| Duration of disease | −0.008 | 1.008 | 0.428 |
| ChT | 0.052 | 1.054 | 0.012 |
BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; ChT: chitotriosidase.
Bold values indicate a p value <0.05.
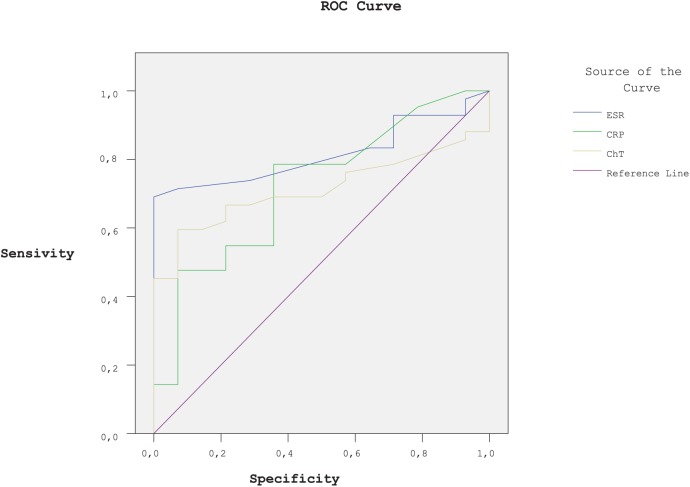
We also used ROC analysis to evaluate the degree of usefulness of ESR, CRP, and serum ChT for assessing disease activity. ESR, CRP, and serum ChT showed the best and significant area under the curve (AUC), when distinguishing the AS patients with higher score of BASDAI (AUC values were 0.815, 0.722, and 0.713, respectively, all p < 0.05; Figure 2).
Figure 2.
ROC curves analysis: comparison of ESR, CRP and serum ChT in demonstrating of active AS patients. [BASDAI ⩾4 as being positive for high disease activity ; area under the curve (AUC) (p value) are 0.815 (<0.05), 0.722 (<0.05), and 0.713 (<0.05), respectively.] Null hypothesis: true area = 0.5.
AS, ankylosing spondylitis; AUC, area under the curve; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; ChT, chitotriosidase; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; ROC, receiver operating characteristic.
Discussion
Our results revealed that serum ChT and neopterin levels were significantly higher in patients with active AS. Moreover, the significant correlation of ChT activity with clinical disease activity and acute-phase response parameters could be interpreted that serum ChT might be a significant predictor for disease activity in AS patients. However, neopterin did neither correlate with clinical disease activity nor with acute-phase response parameters in patients with AS. Higher disease activity was associated with ChT.
ChT has been considered as a marker of macrophage activation, and ChT secretion in chronic inflammatory diseases is a popular scientific subject, which is still not fully understood and to our knowledge, this is the first study showing a relation with ChT levels in patients with AS. Patients with active AS showed significantly higher levels of ChT when compared with patients with inactive AS. ChT is selectively expressed in chronically activated tissue macrophages and reflects chronic inflammation rather than acute inflammation.4 In the present study, ChT showed a positive significant correlation with clinical disease activity and acute-phase response parameters. Thus, serum ChT levels can be helpful not only in estimating the long-term course of AS disease, but also in predicting short-term disease activity levels. A previous study of ChT in juvenile idiopathic arthritis (JIA) was performed to define the relationship between serum ChT level and disease activity.10 This found that there was no correlation between serum ChT, and the clinical disease activity and inflammation markers in patients with JIA. The difference may be due to the age range in this study, as it was juvenile patients.
Neopterin is produced mainly in macrophages and T helper (Th1) lymphocytes derived from interferon gamma (IFN-γ).19 Neopterin concentrations are increased in the body fluids of patients with an activated cellular immune response, such as polycystic ovary syndrome,11 rheumatoid arthritis,12 autoimmune diseases,13,14 Crohn’s disease and ulcerative colitis.15 Previous studies of neopterin in rheumatoid arthritis have been performed to define the relationship between neopterin and disease activity. Generally, they found that neopterin correlated with disease activity in patients with rheumatoid arthritis .20–22 In contrast with the previous studies, our study showed that there was no significant correlation between serum neopterin, and the clinical disease activity and acute-phase response parameters in patients with AS. The difference in the results may be attributed to the pathogenesis of various diseases.
Arshadi and colleagues23 detected significantly higher levels of neopterin in patients with rheumatoid arthritis compared with healthy controls, and found that there was a higher neopterin level in male patients with rheumatoid arthritis than female patients, and a significant correlation of plasma levels of neopterin with age in both the rheumatoid arthritis and control groups. Previous studies have showed an increase in neopterin levels with increasing age.24,25 However, contrary to the previous results, we did not find any significant association between age, medication and duration of disease, and serum level of ChT and neopterin.
The measurement of the levels of the acute-phase reactants appears to have limited value in determining disease activity.26 Only 50–70% of patients with active AS will have an increased level of CRP and ESR.27 Similar to these results, in our study we found that 67% of the patients with active AS had an increased level of ESR, and 79% of the patients with active AS had an increased level of CRP. Yildirim and colleagues showed a significant relationship of BASDAI with only CRP but not with other acute-phase reactants such as ESR, haptoglobin (Hp), beta-2-microglobulin.28 In the present study, we grouped patients as inactive and active according to BASDAI scores <4 or ⩾4 and showed that the acute-phase response markers were significantly higher in patients with active AS. We also showed a positive significant correlation between serum ChT and BASDAI scores.
The present study has several limitations. First, the effects of the type of therapy on serum neopterin and ChT levels were not followed, so their use as a marker for therapy efficacy could not be evaluated. Second, there was a lack of repeated measures in the follow-up period. Third, a potent association with B27 is axial inflammation in the bone, cartilage, and enthesis. The role of B27 is in an immune response that initiates or perpetuates this inflammation. HLA-B27 was not considered in this study. Fourth, assessment of the disease activity was only based on BASDAI scores. The Ankylosing Spondylitis Disease Activity Score in addition to BASDAI might have been used to strengthen the results of the present study. Finally, this study was cross-sectional and did not have a healthy control group.
Conclusion
The present study emphasized that serum ChT levels can be useful in the determination of disease activity in patients with AS. Further studies are needed to assess the relationship between serum neopterin levels and AS activity.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD: Bilge Kesikburun  https://orcid.org/0000-0001-6110-2252
https://orcid.org/0000-0001-6110-2252
Contributor Information
Ferdi Yavuz, Department of Physical Medicine and Rehabilitation, Health Sciences Faculty, European University of Lefke, Gemikonagi, Turkish Republic of Northern Cyprus-Mersin Fizyocare Physical Therapy Center, Alacaatlı cad. 2857 sok. No:7, 06810, Ankara, Turkey.
Bilge Kesikburun, University of Health Sciences, Dışkapı Yıldırım Beyazıt Research and Training Hospital, Department of Physical Medicine and Rehabilitation, Ankara, Turkey.
Özlem Öztürk, Gülhane Research and Training Hospital, Department of Biochemistry, Ankara, Turkey.
Ümüt Güzelküçük, University of Health Sciences, Gülhane School of Medicine, Department of Physical Medicine and Rehabilitation, Ankara, Turkey.
References
- 1. Sieper J, Braun J, Rudwaleit M, et al. Ankylosing spondylitis: an overview. Ann Rheum Dis 2002; 61(Suppl. 3): 8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hollak CE, van Weely S, van Oers MH, et al. Marked elevation of plasma chitotriosidase activity. A novel hallmark of Gaucher disease. J Clin Invest 1994; 93: 1288–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boot RG, Renkema GH, Strijland A, et al. Cloning of a cDNA encoding chitotriosidase, a human chitinase produced by macrophages. J Biol Chem 1995; 270: 26252–26256. [DOI] [PubMed] [Google Scholar]
- 4. Malaguarnera L. Chitotriosidase: the yin and yang. Cell Mol Life Sci 2006; 63: 3018–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Murr C, Widner B, Wirleitner B, et al. Neopterin as a marker for immune system activation. Curr Drug Metabol 2002; 3: 175–187. [DOI] [PubMed] [Google Scholar]
- 6. Guo Y, He W, Boer AM, et al. Elevated plasma chitotriosidase activity in various lysosomal storage disorders. J Inherit Metab Dis 1995; 18: 717–722. [DOI] [PubMed] [Google Scholar]
- 7. Czartoryska B, Tylki-Szymanska A, Lugowska A. Changes in serum chitotriosidase activity with cessation of replacement enzyme (cerebrosidase) administration in Gaucher disease. Clin Biochem 2000; 33: 147–149. [DOI] [PubMed] [Google Scholar]
- 8. Michelakakis H, Dimitriou E, Labadaridis I. The expanding spectrum of disorders with elevated plasma chitotriosidase activity: an update. J Inherit Metab Dis 2004; 27: 705–706. [DOI] [PubMed] [Google Scholar]
- 9. Brunner J, Scholl-Burgi S, Prelog M, et al. Chitotriosidase as a marker of disease activity in sarcoidosis. Rheumatol Int 2007; 27: 1185–1186. [DOI] [PubMed] [Google Scholar]
- 10. Brunner JK, Scholl-Bürgi S, Hössinger D, et al. Chitotriosidase activity in juvenile idiopathic arthritis. Rheumatol Int 2008; 28: 949–950. [DOI] [PubMed] [Google Scholar]
- 11. Alanbay I, Mutlu Ercan C, Çoksuer H, et al. Neopterin: a promising marker for the inflammation in polycystic ovary syndrome. Gynecol Endocrinol 2012; 28: 879–883. [DOI] [PubMed] [Google Scholar]
- 12. D’agostino LE, Ventimiglia F, Verna JA, et al. Correlation between DAS-28 and neopterin as a biochemical marker of immune system activation in early rheumatoid arthritis. Autoimmunity 2013; 46: 44–49. [DOI] [PubMed] [Google Scholar]
- 13. Schroecksnadel K, Murr C, Winkler C, et al. Neopterin to monitor clinical pathologies involving IFN-γ production. Pteridines 2004; 15: 75–90. [Google Scholar]
- 14. Hoffmann G, Wirleitner B, Fuchs D. Potential role of immune system activation-associated production of neopterin derivatives in humans. Inflamm Res 2003; 52: 313–321. [DOI] [PubMed] [Google Scholar]
- 15. Husain N, Tokoro K, Popov JM, et al. Neopterin concentration as an index of disease activity in Crohn’s disease and ulcerative colitis. J Clin Gastroenterol 2013; 47: 246–251. [DOI] [PubMed] [Google Scholar]
- 16. van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 1984; 27: 361–368. [DOI] [PubMed] [Google Scholar]
- 17. Garrett S, Jenkinson T, Kennedy LG, et al. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI). J Rheumatol 1994; 21: 2286–2291. [PubMed] [Google Scholar]
- 18. Fuchs D, Weiss G, Wachter H. Neopterin, biochemistry and clinical use as a marker for cellular immune reactions. Int Arch Allergy Immunol 1993; 101: 1–6. [DOI] [PubMed] [Google Scholar]
- 19. Hamerlinck FF. Neopterin: a review. Exp Dermatol 1999; 8: 167–176. [DOI] [PubMed] [Google Scholar]
- 20. Schroecksnadel K, Frick B, Kaser S, et al. Moderate hyperhomocysteinaemia and immune activation in patients with rheumatoid arthritis. Clin Chim Acta 2003; 338: 157–164. [DOI] [PubMed] [Google Scholar]
- 21. Nasonov EL, Samsonov MIu Tilz G, et al. Neopterin: new immunological marker of autoimmune rheumatic disease. Klin Med (Mosk) 2000; 78: 43–46. [PubMed] [Google Scholar]
- 22. Özkan Y, Mete G, Sepici-Dinçel A, et al. Tryptophan degradation and neopterin levels in treated rheumatoid arthritis patients. Clin Rheumatol 2012; 31: 29–34. [DOI] [PubMed] [Google Scholar]
- 23. Arshadi D, Nikbin B, Shakiba Y, et al. Plasma level of neopterin as a marker of disease activity in treated rheumatoid arthritis patients: association with gender, disease activity and anti-CCP antibody. Int Immunopharmacol 2013; 17: 763–767. [DOI] [PubMed] [Google Scholar]
- 24. Murr C, Hainz U, Asch E, et al. Association of increased neopterin production with decreased humoral immunity in the elderly. Exp Gerontol 2003; 38: 583–587. [DOI] [PubMed] [Google Scholar]
- 25. Frick B, Schroecksnadel K, Neurauter G, et al. Increasing production of homocysteine and neopterin and degradation of tryptophan with older age. Clin Biochem 2004; 37: 684–687. [DOI] [PubMed] [Google Scholar]
- 26. Ruof J, Stucki G. Validity aspects of erythrocyte sedimentation rate and C-reactive protein in ankylosing spondylitis: a literature review. J Rheumatol 1999; 26: 966–970. [PubMed] [Google Scholar]
- 27. Spoorenberg A, van der Heijde D, de Klerk E, et al. Relative value of erythrocyte sedimentation rate and C-reactive protein in assessment of disease activity in ankylosing spondylitis. J Rheumatol 1999; 26: 980–984. [PubMed] [Google Scholar]
- 28. Yıldırım K, Erdal A, Karatay S, et al. Relationship between some acute-phase reactants and the Bath Ankylosing Spondylitis Disease Activity Index in patients with ankylosing spondylitis. South Med J 2004; 97: 350–353. [DOI] [PubMed] [Google Scholar]