Abstract
Background/purpose
There is a variety of pathological alterations occurring in the oral cavity are strongly associated with chronic kidney disease (CKD) or CKD therapy. The aim of this study is to conduct a retrospective analysis to examine the possible correlation between the dental restorative treatment modalities and the progression of kidney disease in CKD population.
Materials and methods
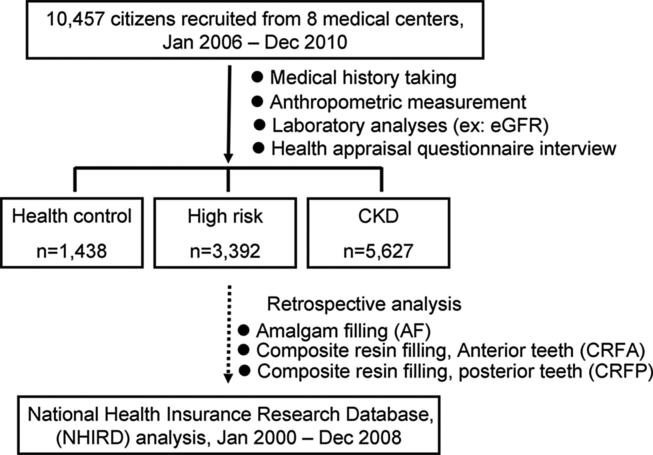
A total of 10,457 individuals were divided into three groups: (HC) group (n = 1438), high risk (HR) group (n = 3392), and CKD group (n = 5627). HR group were defined for those with an eGFR ≥60 (mL/min/1.73 m2) in addition to fulfilling one of the following requirements: (1) being diagnosed diabetes mellitus (DM), hypertension, or cardiovascular disease; (2) having a family member diagnosed with CKD or receiving dialysis treatment. Demographic characteristics, dental restorative treatment utilization and expenditures, including amalgam filling, composite resin filling on anterior teeth or posterior teeth, were analyzed retrospectively (2000–2008) among these groups using a nationwide database.
Results
The utilization and expenditures for various restorative treatments were significantly different among investigated groups, and the health insurance usage exhibited an inverse relationship with CKD stages, especially at CKD stages 4 and 5. A sustained decline in utilization and expenditures for restorative treatment was associated with the deterioration of kidney function. The lowest usage of these restorative modalities was noted in the CKD group and a marked difference was noted among investigated groups.
Conclusion
The findings do, however, provide indirect evidence that if patients with progressive renal failure and receive less dental care.
Keywords: dental insurance, dental care, dental restoration, dental amalgam
Introduction
Chronic kidney disease (CKD), a gradual loss of kidney function over time, has become a national public health problem because of its widespread prevalence in the past decade.1, 2 CKD commonly is affiliated with significant economic burdens to patients and has become a major challenge for healthcare systems.1, 2 Nevertheless, in view of healthcare-related expenditures, comprehensive CKD treatment has been reported to be cost effective: slowing the development and progression of disease and associated complications.1, 3
A growing evidence has indicated that there is a variety of pathological alterations occurring in the oral cavity are strongly associated with CKD or CKD therapy.4, 5 Although the exact causal associations between diseases is complicated,5 it is widely accepted that CKD can affect the oral health status; likewise, poor oral health has influence on the progression of CKD.4, 5, 6, 7 Accordingly, these findings would justify an greater attention and awareness to dental care in CKD patients.4, 8
As maintaining oral health allows for a better clinical and economic prospect for CKD patients; comprehensive evaluation and management for patients by specific dental specialists, such as periodontists and endodontists is highly recommended.5, 6, 7 However, there is limited information discussing the association between the effects of restorative dental treatments on the estimated glomerular filtration rate (eGFR) and kidney function. Moreover, recent publications on healthcare expenditures in CKD have concentrated mainly on hospitalization costs9, 10, 11 and individual co-morbidities12, 13, 14 rather than dental care. Apart from our previous study investigating the possible correlation between utilization of dental services and CKD outcomes;8 no other links between the dental restorative treatment modalities and the progression of kidney disease in CKD population have been studied. Furthermore, there are no existing large, and well-designed population-based studies that provide better scientific evidence to discuss the causation of dental restorative treatment expenditures and resource utilization for CKD patients. This study aims to examine utilization and expenditure for restorative dental treatment modalities in CKD patients through conducting a retrospective claims database analysis.
Materials and methods
Data source and validation
This nationwide hospital-based study recruited individuals from National Health Insurance Research Database (NHIRD), released by the National Health Research Institutes (NHRI). Taiwan launched its National Health Insurance Program (NHIP) on 1st March 1995, and by 2009, a coverage rate of the program had reached 99% of the population.8, 15 The NHIRD contains complete medical information of all insured individuals, including diagnoses, healthy services and claims records for reimbursement. The Bureau of NHI regular justifies and validates medical charts to ensure the accuracy of diagnosis coding system in the NHIRD. Thus, a high fidelity of coding in the NHIRD is considered, and the NHIRD provides a promising statistical representation of data for analyzing epidemiological profiles of the entire Taiwanese population. The NHIRD has been used in several high-quality international peer reviewed journal articles regarding the CKD patients in Taiwan, supporting its validity for research studies.8, 16
Study design
For this study, a total of 10,457 subjects within the NHIP database between 1st January 2006 and 31st December 2010 were analyzed. Study participants were randomly selected and a face-to-face interview was performed to collect the information including present health status, past medical history and family medical history (e.g., diabetes mellitus, cardiovascular diseases, hypertension, cerebrovascular diseases, CKD). Physical examination and anthropometric measurements (e.g., body weight, height, body mass index, waist circumference), and laboratory examination (e.g., estimated glomerular filtration rate) were also performed. Additionally, a demographic questionnaire eliciting information about socio-economic and oral behavioral risk factors (e.g., cigarette smoking, betel nut chewing, and alcohol consumption) was also collected. This study was conducted in full accordance with the World Medical Association Declaration of Helsinki, and the ethics approval for this study was given by the Institutional Review Board of Tri-Service General Hospital, National Defense Medical Center (TSGHIRB 097-05-119). Written informed consent was received from participants before their inclusion in the study.
Definition of participants
The present and previous medical history of each participant was collected by calibrated and well-trained investigators. The phlebotomy was performed by medical scientist to collect blood specimens from each study participant, then send to laboratory for analyzing and calculating the estimated glomerular filtration rate (eGFR) in accordance to the Modification of Diet in Renal Disease study equation.17 Based on the information gathered from reviewing medical history and reports of laboratory analyses, three groups were further categorized: “Healthy control (HC)”, “High risk (HR)”, and “Chronic kidney disease (CKD)”.
For HC group, these subjects were recruited from health evaluation units or communities around participating medical centers for annual routine health examination. Individual with an eGFR ≥60 (mL/min/1.73 m2), and denied medical history with renal-related disorders and associated family members should have no history of renal diseases.
HR group were defined for those with an eGFR ≥60 (mL/min/1.73 m2) in addition to fulfilling one of the following requirements: (1) being diagnosed diabetes mellitus (DM), hypertension, or cardiovascular disease; (2) having a family member diagnosed with CKD or receiving dialysis treatment.
The CKD stages were defined according to the US National Kidney Foundation's 2009 guideline,18 with subdivision of stage 3 into stage 3a (eGFR 45–59 ml/min/1.73 m2) and stage 3b (eGFR 30–44 ml/min/1.73 m2).19
Dental restorative treatment modalities utilization and expenditures
In Taiwan, the prevalence of end-stage renal disease (ESRD) reached 2584 per million in 2010, and treatment of ESRD places an enormous financial burden on our country.20 As retaliation, the Taiwanese government launched a project of multidisciplinary care for CKD patients in 2004, which includes dental care. This service for CKD patients is available throughout Taiwan and is also covered by the NHI program.
In this study, these patients' first ambulatory care visits for dental restorative treatment between 1st January, 2000 and 31st December, 2008 were assigned as the index date use of healthcare. At the end of the four-year study, every participants' claims data regarding their dental restorative treatment utilization and expenses, particularly in amalgam filling (AF) and composite resin filling (CR), were obtained retrospectively from NHIRD for further analysis (Fig. 1).
Figure 1.
Flow chart of the selection process of the study participants.
Potential confounders
The OPD prescription and therapeutic coding system for DENT (40–49) of each participant was retrieved and transcribed from NHIRD. The expenditures and utilization for dental services were defined according to diagnoses in the NHIRD, which are coded using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) coding scheme. The dental restorative procedure codes were defined as: amalgam filling (AF; ICD-9-CM code: 5210–5219; therapeutic code: 89001–8903); anterior teeth composite resin filling (ICD-9-CM code: 5210–5219; therapeutic code: 89004, 89005, and 89012); and posterior teeth composite resin filling (ICD-9-CM code: 5210–5219; therapeutic code: 89008, 89009, and 89010). The dataset after merging with NHIRD was further transcribed for further statistical analysis.
Statistical analyses
Statistical analyses were performed using the SAS 9.13 system (SAS system for windows, version 8.2. SAS Institute Inc. Cary, NC) and SPSS 18.0 software package (SPSS Inc., Chicago, Illinois). When appropriate, the study groups were described characteristically by their mean expenditures and frequency of medical care visits. Through the chi-square test and one-way analysis of variance (ANOVA), statistical differences in categorical variables and continuous variables between the three groups were determined respectively. Statistical significance level was set at p < 0.05.
Results
The selection process and recorded information of the 10,457 eligible participants were illustrated (Fig. 1). The study participants comprised three groups: health control group (HC, n = 1438), high risk group (HR, n = 3392), and CKD group (CKD, n = 5627) were further categorized based on their clinical diagnosis. The demographic characteristics (e.g., gender, age, residential region area) and socioeconomic status (e.g., education level, occupation, household income) exhibited significant differences among all groups (all p < 0.001) (Table 1). Regarding socioeconomic status, the CKD group had higher unemployment rate (56.7%), lower household income (≤40,000 NT$, 71.8%), and lower education achievement (<college level, 84.3%) in comparison to other groups (Table 1).
Table 1.
Demographic data and socioeconomic status of eligible subjects.
| Parameters/Groups | HC (n = 1438) |
HR (n = 3392) |
CKD (n = 5627) |
pa | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Gender | <0.001 | ||||||
| Male | 477 | 33.2 | 1554 | 45.8 | 3247 | 57.7 | |
| Female | 961 | 66.8 | 1838 | 54.2 | 2380 | 42.3 | |
| Age (yrs) | <0.001 | ||||||
| mean ± SD | 46.62 ± 15.15 | 57.59 ± 14.30 | 61.04 ± 15.21 | ||||
| <45 | 680 | 47.3 | 616 | 18.2 | 796 | 14.1 | |
| 45–64 | 551 | 38.3 | 1589 | 46.8 | 2285 | 40.6 | |
| 65–74 | 138 | 9.6 | 794 | 23.4 | 1386 | 24.6 | |
| >75 | 69 | 4.8 | 393 | 11.6 | 1160 | 20.6 | |
| Residential area of Taiwan | <0.001 | ||||||
| Northern | 619 | 43.0 | 1206 | 35.6 | 2419 | 43.0 | |
| Central | 413 | 28.7 | 1127 | 33.2 | 1373 | 24.4 | |
| Southern | 406 | 28.3 | 1059 | 31.2 | 1835 | 32.6 | |
| Education level | <0.001 | ||||||
| <Junior high (%) | 267 | 18.6 | 1442 | 42.5 | 2864 | 50.9 | |
| Senior high (%) | 598 | 41.6 | 1323 | 39.0 | 1879 | 33.4 | |
| >College (%) | 572 | 39.8 | 628 | 18.5 | 883 | 15.7 | |
| Occupation | <0.001 | ||||||
| None | 362 | 25.2 | 1638 | 48.3 | 3191 | 56.7 | |
| Government | 104 | 7.2 | 149 | 4.4 | 242 | 4.3 | |
| Agriculture | 11 | 0.8 | 64 | 1.9 | 135 | 2.4 | |
| Business | 135 | 9.4 | 319 | 9.4 | 445 | 7.9 | |
| Labor | 121 | 8.4 | 282 | 8.3 | 405 | 7.2 | |
| Other | 705 | 49 | 940 | 27.7 | 1210 | 21.5 | |
| Household income (NT$) | <0.001 | ||||||
| None (%) | 224 | 15.6 | 1238 | 36.5 | 2481 | 44.1 | |
| <40,000 (%) | 387 | 26.9 | 987 | 29.1 | 1559 | 27.7 | |
| 4–90,000 (%) | 520 | 36.2 | 814 | 24.0 | 1092 | 19.4 | |
| >90,000 (%) | 306 | 21.3 | 353 | 10.4 | 495 | 8.8 | |
Note: Unless otherwise indicated, values are number (percentage). The eligible subjects were recruited patient from 2008 to 2010. N = 10,457.
Abbreviations: CKD, chronic kidney disease; HC, healthy control; HR, high risk; NT$, New Taiwan dollar.
Chi-square test. p < 0.05 was considered statistically significant.
The dental restorative treatment modalities of each group were analyzed according to the type of restorative materials (amalgam vs. composite resin) and type of filled teeth (anterior vs. posterior) (Table 2). Prevalently utilized restorative procedures, beginning with the most common were: composite resin filling in posterior teeth (CRFP), composite resin filling in anterior teeth (CRFA), and amalgam filling (AF) (Table 2). CKD subjects possessed the lowest rate of dental restorative utilization and expenditure, including amalgam and composite resin filling, in comparison to HC and HR groups (Table 2). Of note, only the CRFP demonstrated significant differences in outpatient visits and expenditures among the investigated groups, in which the HC group was greater than the HR group, and then the CKD group (p < 0.0001) (Table 2).
Table 2.
The analysis of dental restorative treatment modalities including amalgam filling (AF), composite resin filling on anterior (CRFA) and posterior teeth (CRFP) by annual number of outpatient visits, and expenditures per person of eligible patients from 2000 to 2008.
| Parameters/Groups | HC (n = 1438) | HR (n = 3392) | CKD (n = 5627) | pb |
|---|---|---|---|---|
| Amalgam filling (AF) | ||||
| OPD visits/person (mean ± SD) | 0.31 ± 0.27 | 0.32 ± 0.63 | 0.31 ± 0.29 | 0.257 |
| OPD expenditures/persona (NT$, mean) | 3485 | 3574 | 3279 | 0.0474 |
| Composite resin filling, anterior teeth (CRFA) | ||||
| OPD visits/person (mean ± SD) | 0.38 ± 0.36 | 0.4 ± 0.36 | 0.39 ± 0.36 | 0.364 |
| OPD expenditures/persona (NT$, mean) | 5394 | 5508 | 5218 | 0.229 |
| Composite resin filling, posterior teeth (CRFP) | ||||
| OPD visits/person (mean ± SD) | 0.61 ± 0.53 | 0.56 ± 0.51 | 0.5 ± 0.46 | <0.0001 |
| OPD expenditures/persona (NT$, mean) | 9584 | 8378 | 7347 | <0.0001 |
Note: The eligible subjects were recruited patient from 2008 to 2010. N = 10,457.
Abbreviations: CKD, chronic kidney disease; HC, healthy control; HR, high risk NT$, New Taiwan dollar; OPD, outpatient.
All outpatient expenditures (NT$) were rounded to the nearest whole dollar amount.
Chi-square test. p < 0.05 was considered statistically significant.
For all restorative treatments at different CKD stages, the annual utilization and expenditures per person generally decreased as the patients' kidney function became worse, most prominently at CKD stage 5. Notably, the biggest decline was in CRFP (p < 0.0001) for CKD patients (Table 3).
Table 3.
The analysis of dental restorative treatment procedures including amalgam filling, composite resin filling on anterior and posterior teeth by annual number of outpatient visits, and expenditures per person of CKD patients at different stages from 2000 to 2008.
| Parameters/Groups | Stage 1 | Stage 2 | Stage 3a | Stage 3b | Stage 4 | Stage 5 | pb |
|---|---|---|---|---|---|---|---|
| Amalgam filling (AF) | |||||||
| OPD visits/person (mean ± SD) | 0.37 ± 0.38 | 0.32 ± 0.26 | 0.29 ± 0.26 | 0.31 ± 0.3 | 0.31 ± 0.28 | 0.27 ± 0.24 | 0.0009 |
| OPD expenditures/persona (NT$, mean) | 3831 | 3413 | 2994 | 3178 | 3164 | 2866 | 0.0029 |
| Composite resin filling, anterior teeth (CRFA) | |||||||
| OPD visits/person (mean ± SD) | 0.39 ± 0.37 | 0.37 ± 0.32 | 0.42 ± 0.37 | 0.42 ± 0.37 | 0.42 ± 0.41 | 0.34 ± 0.329 | 0.006 |
| OPD expenditures/persona (NT$, mean) | 5242 | 5012 | 5719 | 5470 | 5450 | 4578 | 0.0447 |
| Composite resin filling, posterior teeth (CRFP) | |||||||
| OPD visits/person (mean ± SD) | 0.59 ± 0.49 | 0.52 ± 0.48 | 0.49 ± 0.43 | 0.5 ± 0.44 | 0.48 ± 0.44 | 0.4 ± 0.37 | <0.0001 |
| OPD expenditures/persona (NT$, mean) | 8858 | 7881 | 7205 | 7200 | 6763 | 5781 | <0.0001 |
Note: The eligible subjects were recruited patients from 2008 to 2010. N = 10,457.
Abbreviations: CKD, chronic kidney disease; HC, healthy control; HR, high risk NT$, New Taiwan dollar; OPD, outpatient.
All outpatient expenditures (NT$) were rounded to the nearest whole dollar amount.
Chi-square test. p < 0.05 was considered statistically significant.
Discussion
Dental restorative treatment is an essential aspect of the overall dental service for CKD patients in Taiwan. To our knowledge, this is the first long-term, nationwide population-based cohort study to retrospectively investigate the utilization and expenditure of dental restorative treatment for CKD patients with different CKD stages. The main results show: 1) CKD group demonstrated significant differences in demographic characteristics, and socioeconomic status in comparison to HC and HR group (Table 1); 2) regarding dental restorative treatments, lowest utilization and expenditure, including amalgam and composite resin filling, were found in CKD subjects when compared with HC and HR groups (Table 2); 3) the OPD visits and expenditures for restorative treatment for the CKD group decreased significantly according to the progression of CKD stages (Table 3). These findings provide certain insight into the relationship between CKD and dental restorative treatment services.
In this study, we investigated socioeconomic and demographic data, discovering that patients in the CKD group were more likely to be male, unemployed have low income, and are more than 50% likely to have less than a junior high diploma, suggesting CKD patients possess relative lower socioeconomic level than HC and HR patients (Table 1). An individual's socioeconomic level may be associated with oral health habits, such as cigarette smoking, betel nut chewing, or alcohol consumption, which may eventually determine oral health status.8, 21 For CKD patients, their sociodemographic characteristics may be associated with oral health behavior and may reflect, at least in part, their dental treatment attitude, behavior and even treatment needs.8
First, in our study we investigated socioeconomic and demographic data, finding that group CKD was more likely to be male, unemployed or earning a low income, and more than 50% likely to have less than a junior high diploma. A US study had similar findings, in that people with CKD and limited education or low income have more risk of disability because of socioeconomic disparities.22 Moreover, patients in our CKD group were more likely to have bad oral habits than were other groups (Table 2). A cross-sectional study regarding the oral health status of adults in Taiwan found that demographic factors (i.e., gender, marital status, and income levels) are all significantly associated with general health.23 Thus, our findings highlight the need for more attention to DENT needs for CKD patients.
It has been shown oral hygiene, gingival, and periodontal status were decreased as the stage of CKD increased and was worse among study subjects that the controls.24 For the prevalence of caries in CKD patients, marked alterations in dental conditions, such as dental caries, were significantly changed among study subjects than the controls and were strongly correlated with the duration of kidney disorders.5, 25 Furthermore, dental caries did not differ significantly with the stage of the renal disease.24 In our study, the utilization and expenditures of dental restorative therapy were significantly associated with decreased kidney function (Table 3). However, in this study, our results could only reflect that dental caries treatment needs were gradually decreased as the patients' kidney function became worsen because the exact caries status of each participant was not examined by dentist.
A possible explanation for these results is their saliva; decreased salivary flow rates were relatively prevalent in patients with advanced deterioration of kidney function,26, 27 and were correlated with more dental caries.27, 28 Interestingly, contrary to our expectation, children with kidney disorders were less affected than health individuals in the control group by dental caries because children possess decreased levels of cariogenic microorganisms and increased concentrations of antibacterial chemicals (i.e., urea).29
It is more likely that as renal disease progresses to renal failure and dialysis may be initiated,30 dental health will deteriorate; patients will become more susceptible to dental caries, and will require more dental care.27, 28 Nevertheless, the present study found that significantly fewer dental restorative procedures were performed during the investigation period of time. An alternative explanation for the study findings is that as kidney disease progresses (Table 3), patients become increasingly debilitated and preoccupied with managing their disease, including physician visits, hospitalizations, etc. As a consequence, dental care may not be feasible and becomes less of a priority. Furthermore, dental care may be limited to emergency visits and extractions which may explain the highly significant reduction in composite restoration procedures on posterior teeth. Numerous factors may have a role in deciding the use of dental services, such as dental health literacy, socioeconomic status, geographic barriers, and perhaps financial barriers. However, the exact role of these contributing factors would need further studies and statistical analysis (i.e. multivariate regression analysis) to clarify the impact of deciding the use of dental services of investigated individuals, especially CKD group.
In our study, dental expenditure and utilization may provide contributory information on the deterioration of kidney function in CKD patients (Table 3), suggesting that attention should be especially paid to dental status in patients with advanced CKD status. The current evidence could enhance the understanding that adequate prevention and management of oral diseases may improve future prospects for CKD patients. Further studies of cohorts to ascertain the causal and effect relationship between oral health, such as caries and CKD progression is urgent needed. Thus, for CKD patients, dental health should be targeted for early intervention to limit the impact of oral diseases, improve quality of life, and prevent further encumber nutrient status, all of which could burden CKD outcomes.8
Among those three major restorative procedures, dental amalgam filling was least utilized, particularly in patients with CKD (Table 3), and the usage was inversely associated with CKD progression (Table 3). It could be attributed to NHI policy in Taiwan provides more insurance payment for resin materials than amalgam which may explain, at least in part, the extensive usage of resin filling in dental restorative therapy. Although resin materials are most popular, there are several critical drawbacks: polymerization shrinkage, cracking and microleakage of the fillings.31, 32 In addition, resin-based materials accumulate more dental plaque and become an ecological niche for microorganisms,31, 32, 33 which, in comparison to amalgam, become more cariogenic as composites do not have the antibacterial effects of, compositions in dental amalgam, such as Hg ions.34, 35 Recent evidences demonstrated that composite resin restorations in posterior teeth still have less longevity and a higher number of secondary caries when compared to amalgam restorations.35 On the other hand, detrimental effects of mercury vapor exposure from mercury-containing amalgam fillings may have hazards to health.35, 36 Dental amalgam remains a safe and effective restorative material, capable of providing a reliable solution for carious teeth.35 It should be noted that chronic exposure to mercury vapor may induce an immunological glomerular disease because the kidney is a critical target organ and mercury deposition in kidney increases proportionally with the dose.36 However, on the contrary, a predominance of evidence suggests that mercury-containing amalgam restorative material has caused no renal effects.36, 37 Nevertheless, there is currently a worldwide trend towards replacing amalgam restorations with mercury-free materials, which are adhesive and promote aesthetics.35
In this study, a few limitations still need to be addressed. First, the study is nationwide, population-based control study. However, the study is designed as a cluster randomized without age- or gender-match. The major weakness of this kind of study design is lacking comparability. However, the design allows more accurate sample size increases and expenditure calculations. Second, claims data were identified from the NHIRD under the principal payment code; however, to date, the decision criteria for subjects and their type of restorative treatment modalities, is still judged by clinicians according to individuals' clinical conditions. Third, the study evaluated only the direct dental restorative costs, including amalgam and composite resin filling expenditures, which the claim date could be retrieved from NHIRD. Currently, there was no available information to determine the indirect restorative procedures, such as onlay and inlay, fixed prosthesis, and other prosthodontics treatments. Finally, the present study may also suffer from detection bias. The research design at the very beginning was retrospective examination and comparison of the expenditures and utilization of medical service for recruited participants from 2000 to 2008. The information was transcribed and further analyzed at that time; however, some confounders could not be further analyzed using the regression model because the NHI database was restricted due to privacy issues. We believe that further statistical analysis would have novel findings regarding these issues if some confounders could be further analyzed using the regression model. As information regarding the proportion of self-payment restorative therapies such as crown/bridge fabrication, inlay or onlay restorations, or dental implant placement were not included in the NHIRD, underestimation of expenditures and utilizations may have occurred in this study. Actually, further studies would be urgent needed to clarify the exact causal relationship between use of dental services and progression of renal disease. Therefore, we should be careful and cautions about the interpretation of the results; the interacting effects of these covariates on the correlation between CKD stages and dental restorative treatment utilization and expenditure still require further investigation.
Despite these limitations, this research has several advantages including the importance of reflecting real-world nationwide population-based database, a relatively large sample size, questionnaire retained face-to-face interview with each participant, and the availability of laboratory results to ascertain CKD stages.
Within the limitation of this study, the findings from this study do, however, provide indirect evidence that if patients with progressive renal failure and receive less dental care, this could place them at increased risk for dental infections. This would pose a significant threat if renal transplantation is undertaken and supports the necessity and justification for a pre-transplant dental health evaluation. Increased attention to dental problems may be warranted during the progression of CKD to alleviate the financial and health burden on public healthcare systems; therefore, nephrologists and dentists should implement multi-disciplinary strategies to evaluate and provide a more comprehensive treatment for CKD patients.
Conflicts of interest
The authors have no conflicts of interest relevant to this article.
Acknowledgements
The authors acknowledge Dr. Fu-Gong Lin, and Ms. Jing-Shu Huang (School of Public Health, National Defense Medical Center (N.D.M.C)) and Ms. Hui-Chih Liu (Graduate Institute of Life Sciences, N.D.M.C) for assistance with statistical analysis. The authors also appreciate Dr. Cathy Tsai (School of Dentistry, N.D.M.C), and Professor Mary Goodwin's help in manuscript editing. The authors declare no conflicts of interest related to this study. This study is based in part on data from the NHIRD provided by the Bureau of NHI, Department of Health, managed by National Health Research Institutes in Taiwan and supported by the Ministry of Science and Technology, and Ministry of Health and Welfare, Department of Health of Taiwan under grant DOH97-HP-1101, DOH-98-1110, DOH99-HP-1106, DOH100-HP-1102, DOH101-HP-1103, and DOH102-HP-1103.
References
- 1.Brophy P.D., Shoham D.A., Charlton J.R. Early-life course socioeconomic factors and chronic kidney disease. Adv Chronic Kidney Dis. 2015;22:16–23. doi: 10.1053/j.ackd.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Nicholas S.B., Kalantar-Zadeh K., Norris K.C. Socioeconomic disparities in chronic kidney disease. Adv Chronic Kidney Dis. 2015;22:6–15. doi: 10.1053/j.ackd.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Komenda P., Ferguson T.W., Macdonald K. Cost-effectiveness of primary screening for CKD: a systematic review. Am J Kidney Dis. 2014;63:789–797. doi: 10.1053/j.ajkd.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 4.Ruospo M., Palmer S.C., Craig J.C. Prevalence and severity of oral disease in adults with chronic kidney disease: a systematic review of observational studies. Nephrol Dial Transpl. 2014;29:364–375. doi: 10.1093/ndt/gft401. [DOI] [PubMed] [Google Scholar]
- 5.Akar H., Akar G.C., Carrero J.J., Stenvinkel P., Lindholm B. Systemic consequences of poor oral health in chronic kidney disease patients. Clin J Am Soc Nephrol. 2011;6:218–226. doi: 10.2215/CJN.05470610. [DOI] [PubMed] [Google Scholar]
- 6.Chambrone L., Foz A.M., Guglielmetti M.R. Periodontitis and chronic kidney disease: a systematic review of the association of diseases and the effect of periodontal treatment on estimated glomerular filtration rate. J Clin Periodontol. 2013;40:443–456. doi: 10.1111/jcpe.12067. [DOI] [PubMed] [Google Scholar]
- 7.Ioannidou E., Swede H., Fares G., Himmelfarb J. Tooth loss strongly associates with malnutrition in chronic kidney disease. J Periodontol. 2014;85:899–907. doi: 10.1902/jop.2013.130347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang R.Y., Lin Y.F., Kao S.Y., Shieh Y.S., Chen J.S. A retrospective case-control analysis of the outpatient expenditures for western medicine and dental treatment modalities in CKD patients in Taiwan. PLoS One. 2014;9:e88418. doi: 10.1371/journal.pone.0088418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lei C.C., Lee P.H., Hsu Y.C. Educational intervention in CKD retards disease progression and reduces medical costs for patients with stage 5 CKD. Ren Fail. 2013;35:9–16. doi: 10.3109/0886022X.2012.731997. [DOI] [PubMed] [Google Scholar]
- 10.Villarreal-Rios E., Cardenas-Maldonado C., Vargas-Daza E.R., Galicia-Rodriguez L., Martinez-Gonzalez L., Baca-Baca R. Institutional and familial cost of patients in continuous ambulatory peritoneal dialysis. Rev Assoc Med Bras. 2014;60:335–341. doi: 10.1590/1806-9282.60.04.012. [DOI] [PubMed] [Google Scholar]
- 11.Coyte P.C., Young L.G., Tipper B.L. An economic evaluation of hospital-based hemodialysis and home-based peritoneal dialysis for pediatric patients. Am J Kidney Dis. 1996;27:557–565. doi: 10.1016/s0272-6386(96)90167-5. [DOI] [PubMed] [Google Scholar]
- 12.Jaspers L., Colpani V., Chaker L. The global impact of non-communicable diseases on households and impoverishment: a systematic review. Eur J Epidemiol. 2015;30:163–188. doi: 10.1007/s10654-014-9983-3. [DOI] [PubMed] [Google Scholar]
- 13.Higashiyama A., Okamura T., Watanabe M. Effect of chronic kidney disease on individual and population medical expenditures in the Japanese population. Hypertens Res. 2009;32:450–454. doi: 10.1038/hr.2009.51. [DOI] [PubMed] [Google Scholar]
- 14.Frankenfield D.L., Weinhandl E.D., Powers C.A., Howell B.L., Herzog C.A., St Peter W.L. Utilization and costs of cardiovascular disease medications in dialysis patients in Medicare Part D. Am J Kidney Dis. 2012;59:670–681. doi: 10.1053/j.ajkd.2011.10.047. [DOI] [PubMed] [Google Scholar]
- 15.Yuh D.Y., Cheng G.L., Chien W.C. Factors affecting treatment decisions and outcomes of root-resected molars: a nationwide study. J Periodontol. 2013;84:1528–1535. doi: 10.1902/jop.2013.120580. [DOI] [PubMed] [Google Scholar]
- 16.Wu M.Y., Hsu Y.H., Su C.L., Lin Y.F., Lin H.W. Risk of herpes zoster in CKD: a matched-cohort study based on administrative data. Am J Kidney Dis. 2012;60:548–552. doi: 10.1053/j.ajkd.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 17.Levey A.S., Coresh J., Greene T. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 18.Uhlig K., Berns J.S., Kestenbaum B. KDOQI US commentary on the 2009 KDIGO clinical practice guideline for the diagnosis, evaluation, and treatment of CKD-Mineral and bone disorder (CKD-MBD) Am J Kidney Dis. 2010;55:773–799. doi: 10.1053/j.ajkd.2010.02.340. [DOI] [PubMed] [Google Scholar]
- 19.Go A.S., Chertow G.M., Fan D., McCulloch C.E., Hsu C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 20.Liang C.H., Yang C.Y., Lu K.C. Factors affecting peritoneal dialysis selection in Taiwanese patients with chronic kidney disease. Int Nurs Rev. 2011;58:463–469. doi: 10.1111/j.1466-7657.2011.00913.x. [DOI] [PubMed] [Google Scholar]
- 21.White D.A., Tsakos G., Pitts N.B. Adult Dental Health Survey 2009: common oral health conditions and their impact on the population. Br Dent J. 2012;213:567–572. doi: 10.1038/sj.bdj.2012.1088. [DOI] [PubMed] [Google Scholar]
- 22.Plantinga L.C., Johansen K.L., Schillinger D., Powe N.R. Lower socioeconomic status and disability among US adults with chronic kidney disease, 1999–2008. Prev Chronic Dis. 2012;9:E12. [PMC free article] [PubMed] [Google Scholar]
- 23.Wang T.F., Chou C., Shu Y. Assessing the effects of oral health-related variables on quality of life in Taiwanese adults. Qual Life Res. 2012;22:811–825. doi: 10.1007/s11136-012-0205-8. [DOI] [PubMed] [Google Scholar]
- 24.Tadakamadla J., Kumar S., Mamatha G.P. Comparative evaluation of oral health status of chronic kidney disease (CKD) patients in various stages and healthy controls. Spec Care Dent. 2014;34:122–126. doi: 10.1111/scd.12040. [DOI] [PubMed] [Google Scholar]
- 25.Sekiguchi R.T., Pannuti C.M., Silva H.T., Jr., Medina-Pestana J.O., Romito G.A. Decrease in oral health may be associated with length of time since beginning dialysis. Spec Care Dent. 2012;32:6–10. doi: 10.1111/j.1754-4505.2011.00223.x. [DOI] [PubMed] [Google Scholar]
- 26.Vesterinen M., Ruokonen H., Furuholm J., Honkanen E., Meurman J.H. Oral health in predialysis patients with emphasis on diabetic nephropathy. Clin Oral Investig. 2011;15:99–104. doi: 10.1007/s00784-009-0360-7. [DOI] [PubMed] [Google Scholar]
- 27.Bayraktar G., Kazancioglu R., Bozfakioglu S., Yildiz A., Ark E. Evaluation of salivary parameters and dental status in adult hemodialysis patients. Clin Nephrol. 2004;62:380–383. doi: 10.5414/cnp62380. [DOI] [PubMed] [Google Scholar]
- 28.Thorman R., Neovius M., Hylander B. Clinical findings in oral health during progression of chronic kidney disease to end-stage renal disease in a Swedish population. Scand J Urol Nephrol. 2009;43:154–159. doi: 10.1080/00365590802464817. [DOI] [PubMed] [Google Scholar]
- 29.Ertugrul F., Elbek-Cubukcu C., Sabah E., Mir S. The oral health status of children undergoing hemodialysis treatment. Turk J Pediatr. 2003;45:108–113. [PubMed] [Google Scholar]
- 30.Lin C.M., Yang M.C., Hwang S.J., Sung J.M. Progression of stages 3b-5 chronic kidney disease–preliminary results of Taiwan national pre-ESRD disease management program in Southern Taiwan. J Formos Med Assoc. 2013;112:773–782. doi: 10.1016/j.jfma.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 31.Blum I.R., Lynch C.D., Wilson N.H. Factors influencing repair of dental restorations with resin composite. Clin Cosmet Investig Dent. 2014;6:81–87. doi: 10.2147/CCIDE.S53461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heintze S.D., Rousson V., Hickel R. Clinical effectiveness of direct anterior restorations–a meta-analysis. Dent Mater. 2015;31:481–495. doi: 10.1016/j.dental.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 33.Klimm W., Buchmann G., Reissig D., Schneider H. Verification of microorganisms in dentin of filled teeth by comparison of light, transmission electron and scanning electron microscopy. Stomatol DDR. 1988;38:702–707. [PubMed] [Google Scholar]
- 34.Mo S.S., Bao W., Lai G.Y., Wang J., Li M.Y. The microfloral analysis of secondary caries biofilm around Class I and Class II composite and amalgam fillings. BMC Infect Dis. 2010;10:241. doi: 10.1186/1471-2334-10-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moraschini V., Fai C.K., Alto R.M., Dos Santos G.O. Amalgam and resin composite longevity of posterior restorations: a systematic review and meta-analysis. J Dent. 2015;43:1043–1050. doi: 10.1016/j.jdent.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 36.Guzzi G., Fogazzi G.B., Cantu M. Dental amalgam, mercury toxicity, and renal autoimmunity. J Environ Pathol Toxicol Oncol. 2008;27:147–155. doi: 10.1615/jenvironpatholtoxicoloncol.v27.i2.70. [DOI] [PubMed] [Google Scholar]
- 37.Bates M.N., Fawcett J., Garrett N., Cutress T., Kjellstrom T. Health effects of dental amalgam exposure: a retrospective cohort study. Int J Epidemiol. 2004;33:894–902. doi: 10.1093/ije/dyh164. [DOI] [PubMed] [Google Scholar]