Abstract
Aims/Introduction
We compared the efficacy and safety of insulin glargine 300 U/mL (Gla300) and insulin degludec U100 (Deg) using a flash glucose monitoring system.
Materials and Methods
A total of 24 Japanese patients with type 2 diabetes were randomized to receive once‐daily Gla300 (n = 12) or Deg (n = 12) in the morning. The primary end‐points were the mean percentage of time in the target glucose range (70–179 mg/dL) and hypoglycemia (<70 mg/dL), as measured using flash glucose monitoring during the last 7 days of each 14‐day period.
Results
The percentages of time with glucose levels <70 mg/dL were not significantly different between the two insulin treatments. No significant differences were observed in the percentages of time with glucose levels of 70–179 mg/dL or ≥180 mg/dL. The percentage of time with nocturnal hypoglycemia with Gla300 was significantly lower than that with Deg treatment (P = 0.021). This difference might be attributable to the difference in the duration of action between the two formulations, and the incidence of nocturnal hypoglycemia with Deg treatment was associated with the concomitant use of metformin (P = 0.035).
Conclusions
The two formulations were comparable in efficacy, whereas the incidence of nocturnal hypoglycemia was significantly lower with Gla300. Thus, the present study suggests that, although Gla300 and Deg are comparable long‐acting insulin analogs, Gla300 is safer with respect to the incidence of hypoglycemia.
Keywords: Flash glucose monitoring, Insulin degludec, Insulin glargine 300 U/mL
Introduction
In patients with type 2 diabetes, implementation of strict glycemic control at disease onset is well‐known to be effective for preventing diabetic complications1. However, strict glycemic control has been reported to increase the risk of hypoglycemia2, 3. Severe hypoglycemia and nocturnal hypoglycemia are considered to be important limiting factors in strict glycemic control, and they are risk factors for adverse events, cardiovascular disease and mortality4, 5, 6. Furthermore, hypoglycemia, which can be a serious problem for patients, has been shown to negatively impact treatment outcomes6, 7 and reduce quality of life8, 9. Thus, in the treatment of diabetes, it is critical to achieve favorable glycemic control while preventing hypoglycemia.
Insulin glargine 300 U/mL (Gla300) and insulin degludec U100 (Deg) are long‐acting, once‐daily, basal insulin analogs. Both reduce the risk of hypoglycemia over a 24‐h period, including nocturnal hypoglycemia, compared with insulin glargine 100 U/mL (Gla100), which is the most widely used dose of basal insulin analog at present10, 11, 12, 13. However, only a few studies have been carried out to compare Gla300 and Deg to determine which is more effective and safe. Therefore, in the present study, we compared the efficacy and safety of Gla300 and Deg with respect to glycemic control in type 2 diabetes patients.
Methods
The present single‐center, randomized, open‐label, parallel‐group, two‐period, cross‐over study of patients with type 2 diabetes was conducted from March to July 2017. This study was carried out in accordance with the Declaration of Helsinki (1975, revised in 2013). Before the study, the protocol was approved by the ethics committee of Murakami Memorial Hospital (No. 2017‐2). A total of 24 type 2 diabetes patients who had been treated with Deg in the morning and oral hypoglycemic agents at the outpatient clinic at Murakami Memorial Hospital, Onomichi, Hiroshima, Japan, for ≥3 months were included. All participants provided written informed consent. The protocol is shown in Figure 1. The patients were randomly divided into two groups: Gla300‐Deg and Deg‐Gla300. In the Gla300‐Deg group, the pretrial Deg was replaced with the same dose of Gla300, and a FreeStyle Libre Pro® – a flash glucose monitoring (FGM) system (Abbot Diabetes Care, Chicago, Illinois, USA) – was worn to begin measurements. The doses were adjusted during the first week of wearing the FGM system, and measurements were obtained for 1 week thereafter. The algorithm for dose adjustment was as follows. When the blood glucose level before breakfast was ≥250 mg/dL, the dose was increased by 3 units; when the level was 200–249 mg/dL, the dose was increased by 2 units; when the level was 150–199 mg/dL, the dose was increased by 1 unit; when the level was 100–149 mg/dL, the dose was not changed; and when the level was <100 mg/dL, the dose was decreased by 1 unit. The dose was adjusted daily.
Figure 1.
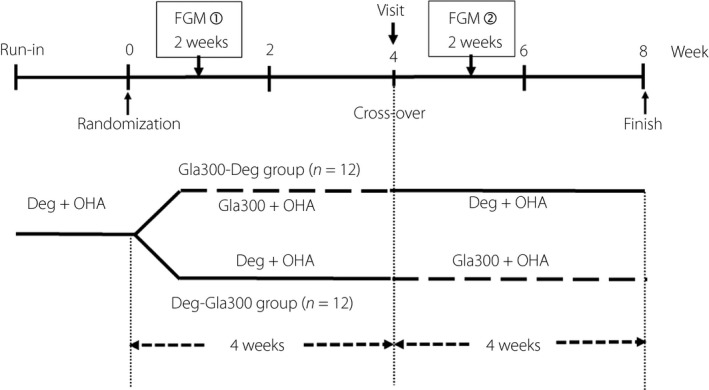
Study design. Deg U100, insulin degludec U100; FGM, flash glucose monitoring; Gla300, insulin glargine 300 U/mL; OHA, oral hypoglycemic agent.
We used a titration that we believed would not cause hypoglycemia, but would be conducive to the experimental timeline. Because the FGM was limited to 14 days and adjustments within that time period were necessary, we carried out daily, rather than weekly adjustments. At an outpatient visit 1 month later, Gla300 was switched to the same dose of Deg, and FGM was restarted. As with Gla300, the Deg dose was adjusted during the first week of monitoring, and measurements were obtained for 1 week thereafter. The Deg‐Gla300 group was treated and monitored in the same manner, but with the dosage schedules reversed. During the study period, the patients received individualized instructions on proper diet and compliance.
Outcome measures and measurements
The primary end‐points of the present study were the efficacy and safety outcomes based on the FGM parameters. The efficacy outcome was calculated as the mean percentage of time within the FGM glucose range of 70–179 mg/dL for the seven consecutive days of each treatment period. The safety outcome was calculated as the mean percentage of time with glucose levels of <70 mg/dL. Secondary end‐points based on FGM included the standard deviation, coefficient of variation, mean glucose level and mean percentage of time with severe hypoglycemia (<54 mg/dL), nocturnal (00.00–05.59 hours) hypoglycemia (<70 mg/dL), and hyperglycemia (≥180 mg/dL) for the seven consecutive days. The mean of daily difference for a 24‐h period was used as an index of day‐to‐day glucose variability. According to the ADA guideline and 2018–2019 Diabetes treatment guide, we defined <70 mg/dL as the hypoglycemic range, <54 mg/dL as the severely hypoglycemic range and 70–179 mg/dL as the normal range14, 15.
Statistical analysis
The data are expressed as the mean ± standard deviation, unless otherwise stated. The findings were compared between the two treatments using Student's t‐tests or χ2‐tests. A P‐value of <0.05 was considered significant for all analyses. Statistical analyses were carried out using JMP 10 software (SAS Institute Inc., Cary, North Carolina, USA).
Results
The overall patient composition is shown in Figure S1. Table 1 shows the patient characteristics. There were no significant differences in any of the patient parameters between the two treatment groups. Figure 2 shows the average daily glucose profiles for the seven consecutive days of measurement using FGM. The glucose variations were similar between the two treatment groups. The percentages of time with blood glucose levels of 70–179 mg/dL (normal range), which indicates effective glycemic control, were 73.4 ± 14.9% with Gla300 treatment and 77.3 ± 11.8% with Deg treatment (Table 2). The percentages of time with blood glucose levels of ≥180 mg/dL (hyperglycemic range) were 26.4 ± 15.1% and 21.1 ± 12.6%, respectively. No significant differences were observed between the two treatments in either parameter. During the 7‐day FGM, the mean blood glucose levels were 153.5 ± 22.2 mg/dL with Gla300 treatment and 146.2 ± 19.3 mg/dL with Deg treatment; the coefficient of variation was 26.0 ± 4.7% and 26.9 ± 5.4%, respectively, and the mean of daily difference was 32.2 ± 13.0 and 35.6 ± 15.9 mg/dL, respectively. No significant differences were observed in any of these parameters. The basal insulin doses did not significantly differ between the two groups.
Table 1.
Baseline characteristics of randomized patients
| Overall (n = 24) | Gla300‐Deg (n = 12) | Deg‐Gla300 (n = 12) | P‐value | |
|---|---|---|---|---|
| Age (years) | 70.7 ± 7.6 | 69.5 ± 9.5 | 71.9 ± 5.2 | 0.447 |
| Duration of diabetes (years) | 14.0 ± 9.3 | 11.6 ± 9.1 | 16.5 ± 9.1 | 0.199 |
| Male, n (%) | 12 (50.0) | 5 (41.7) | 7 (58.3) | 0.436 |
| BMI (kg/m2) | 23.1 ± 3.3 | 24.0 ± 2.4 | 22.3 ± 3.6 | 0.179 |
| HbA1c (%) | 6.80 ± 0.35 | 6.78 ± 0.33 | 6.83 ± 0.34 | 0.780 |
| S‐CPR (ng/mL) | 1.1 ± 0.6 | 1.3 ± 0.7 | 0.9 ± 0.4 | 0.057 |
| Basal insulin dosage (U) | 6.0 ± 3.0 | 5.9 ± 2.5 | 6.2 ± 3.5 | 0.843 |
| Antidiabetic agents | ||||
| DPP4 inhibitor (n) | 20 | 10 | 10 | 0.500 |
| Metformin (n) | 11 | 7 | 4 | 0.313 |
| SGLT2 inhibitor (n) | 6 | 4 | 2 | 0.394 |
| Sulfonylurea (n) | 1 | 1 | 0 | 0.322 |
| Glinides (n) | 16 | 7 | 9 | 0.550 |
| α‐GI | 14 | 5 | 9 | 0.212 |
Values are expressed as mean ± standard deviation. α‐GI, alpha‐glucosidase inhibitor; BMI, body mass index; Deg, insulin degludec U100; DPP4, dipeptidyl peptidase‐4; Gla300, insulin glargine 300 U/mL; HbA1c, glycated hemoglobin; S‐CPR, serum C‐peptide; SGLT2, sodium–glucose cotransporter 2.
Figure 2.
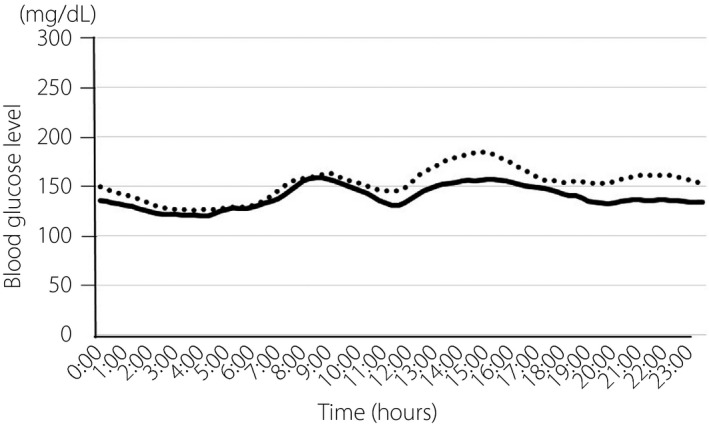
Glycemic variability over 24 h in patients during treatment with insulin glargine 300 U/mL or insulin degludec. Glucose levels were calculated from the flash glucose monitoring on the seventh measurement day. Dotted line, insulin glargine 300 U/mL; solid line, insulin degludec.
Table 2.
Flash glucose monitoring parameters of glucose variability in patients treated with insulin glargine 300 U/mL or degludec U100
| Gla300 (n = 24) | Deg (n = 24) | P‐value | |
|---|---|---|---|
| Mean percentage of time in target glucose range 70–179 mg/dL, (%) | 73.4 ± 14.9 | 77.3 ± 11.8 | 0.314 |
| Mean percentage of time with hyperglycemia ≥180 mg/dL (%) | 26.4 ± 15.1 | 21.1 ± 12.6 | 0.194 |
| Mean percentage of time with hypoglycemia <70 mg/dL (%) | 0.22 ± 0.50 | 1.58 ± 3.93 | 0.100 |
| Mean glucose level (mg/dL) | 153.5 ± 22.2 | 146.2 ± 19.3 | 0.226 |
| SD (mg/dL) | 39.7 ± 8.8 | 40.1 ± 9.1 | 0.810 |
| CV (%) | 26.0 ± 4.7 | 26.9 ± 5.4 | 0.539 |
| MODD (mg/dL) | 32.2 ± 13.0 | 35.6 ± 15.9 | 0.430 |
| Mean percentage of time with severe hypoglycemia <54 mg/dL (%) | 0.01 ± 0.03 | 0.20 ± 0.77 | 0.213 |
| Mean percentage of time with nocturnal hypoglycemia <70 mg/dL (%) | 0.03 ± 0.10 | 0.68 ± 1.34 | 0.021 |
| Mean basal insulin dose (U/day) | 6.2 ± 3.3 | 6.1 ± 3.3 | 0.895 |
Values are expressed as means ± standard deviation. CV, coefficient of variation; Deg, insulin degludec U100; Gla300, insulin glargine 300 U/mL; MODD, mean of daily difference; SD, standard deviation of the glucose levels.
The percentages of time with blood glucose levels <70 mg/dL (hypoglycemic range) were 0.22 ± 0.50% with Gla300 treatment and 1.58 ± 3.93% with Deg treatment, showing no significant difference. The percentages of time with blood glucose levels <54 mg/dL (severe hypoglycemic range) were 0.01 ± 0.03% and 0.20 ± 0.77%, respectively, showing no significant difference. The percentages of time in the nocturnal hypoglycemic range (blood glucose <70 mg/dL at 00.00–05.59 hours) were 0.03 ± 0.10% and 0.68 ± 1.34%, respectively. This percentage was significantly lower with Gla300 treatment than with Deg treatment (P = 0.021).
The hourly frequency of hypoglycemia (event/patient) with Gla300 and Deg treatment was 0.04 and 1.08 (from 00.00 to 00.59 hours), 0 and 0.79 (from 03.00 to 03.59 hours), and 0 and 0.58 (from 04.00 to 04.59 hours). The frequency of hypoglycemia was significantly lower with Gla300 treatment (P < 0.05; Figure 3). When the factors associated with nocturnal hypoglycemia during Deg treatment were investigated, a significant difference was observed in the concomitant use of metformin. Nine patients treated with Deg and metformin developed nocturnal hypoglycemia, whereas two did not (P = 0.035).
Figure 3.
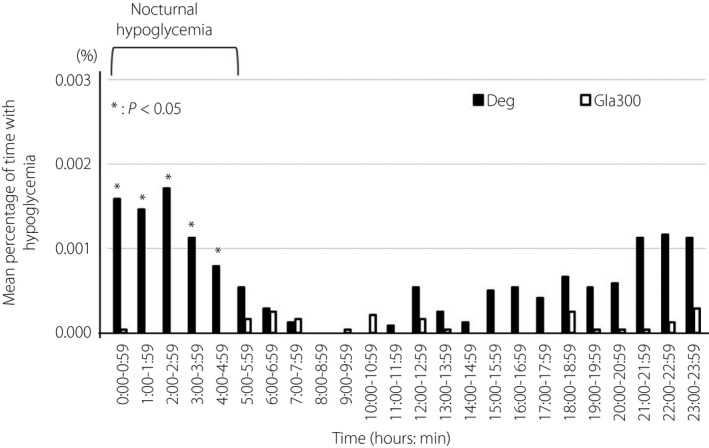
Mean percentage of time at <70 mg/dL measured by flash glucose monitoring. Deg, insulin degludec U100 (black bar); Gla300, insulin glargine 300 U/mL (white bar). *P < 0.05.
In addition, the percentage of time with nocturnal hypoglycemia in the group using metformin (n = 11) was 1.45 ± 1.72%, and that in the group not using metformin (n = 13) was 0.03 ± 0.12%. The time with nocturnal hypoglycemia was significantly higher in the group using metformin (P = 0.007).
No association was observed with the concomitant use of other drugs. With Gla300 treatment, no concomitant drugs were associated with nocturnal hypoglycemia (data not shown).
Discussion
To optimize glycemic control while minimizing the risk of hypoglycemia, basal insulin analogs, with more constant and long‐acting pharmacokinetics and pharmacodynamics, have been developed16, 17. Gla100 is the most commonly used basal insulin analog. Since Gla300 and Deg were approved as long‐acting, once‐daily, basal insulin analogs, many clinical studies have compared the efficacy and safety of Gla300 and Gla100 or of Deg and Gla100. In type 2 diabetes patients treated with either basal supported oral therapy or basal–bolus insulin therapy, Gla300 and Deg have been reported to be associated with a lower risk of hypoglycemia than Gla10018, 19, 20, 21, 22, 23. However, few clinical studies have directly compared the efficacy and safety of Gla300 and Deg.
Using an FGM system, the present study showed that Gla300 and Deg were comparable in efficacy, without significant differences in the percentages of time in the normal and hyperglycemic ranges, mean blood glucose levels, standard deviation, coefficient of variation or day‐to‐day variation. However, with respect to safety, Gla300 tended to be associated with a lower incidence of hypoglycemia than Deg, and the incidence of nocturnal hypoglycemia was significantly lower for Gla300. Differences between Gla300 and Deg have been reported in their mechanism of action24, 25, half‐life26, duration of action16, 27 and fluctuations in blood glucose levels due to glucose clamp28. When we investigated the causes for the higher incidence of nocturnal hypoglycemia with Deg treatment, no differences were observed in age, body mass index, glycated hemoglobin levels, C‐peptide immunoreactivity or basal insulin doses (data not shown). However, although no differences were observed in the Gla300 group in the concomitant use of oral hypoglycemic agents, the Deg group showed a significant difference between patients treated with and without metformin. Metformin is known to inhibit hepatic gluconeogenesis29, 30. It has been reported that Deg has a half‐life of 25 h and a duration of action of >42 h26, 27, whereas Gla300 has a half‐life of 18 h and a duration of action of >24 h16, 26. In the present study, because both formulations were administered in the morning, the difference in duration of action presumably contributed to the difference in the incidence of nocturnal hypoglycemia. Specifically, we assumed that nocturnal hypoglycemia occurred in the Deg group, because residual Deg activity and metformin inhibition of hepatic gluconeogenesis exacerbated night‐time glucose homeostasis. In the present study, we could not measure gluconeogenesis during the night, so this remains a speculation.
Rather than standard weekly adjustments, our insulin titrations utilized daily adjustments to allow optimization within the limited, 14‐day time‐period of the FGM. However, the average number of units of insulin was very small in both groups. In addition, no significant difference was observed between the number of units of insulin administered at the beginning and the end of the study. Therefore, it is unlikely that the frequency of hypoglycemia increased due to daily insulin adjustment. During the study period, no hypoglycemic symptoms were reported by any patient in either group.
The present study had several limitations. The first limitation is that this was a single facility, open label study. The second limitation is that low glucose events were confirmed only by FGM and not by another device, raising the possibility that the actual blood glucose level was not low. Because there was another report of the occurrence of hypoglycemia in asymptomatic patients measured using continuous glucose monitoring31, regular monitoring of night‐time blood glucose levels is important even for patients with well‐controlled blood glucose. Although long‐acting insulin analogs stabilize blood glucose levels and provide favorable glycemic control through their extended duration of action, this prolonged activity might also adversely affect night‐time blood glucose levels. The present study indicates that the drugs used in combination with Deg should receive more consideration. Despite the study limitations, we believe that important information can be garnered from the data, which warrant, at least, further study.
In the present randomized cross‐over study using an FGM system, we compared and analyzed the efficacy and safety of Gla300 and Deg in type 2 diabetes patients. Although these two insulin formulations were comparable in efficacy, Gla300 was safer than Deg in terms of the incidence of nocturnal hypoglycemia, especially with concomitant metformin treatment. This difference seems to be caused by the difference in pharmacodynamics of each drug. In the case of Deg, when metformin is used in combination, the present data suggest that attention should be paid to night‐time hypoglycemia, of which the patient might be unaware.
Disclosure
M Yamabe has received honoraria for lectures from Novo Nordisk and Sanofi. The other authors declare no conflict of interest.
Supporting information
Figure S1¦ Flow diagram of study participants. The numbers in parentheses indicate the numbers of study participants at each step.
J Diabetes Investig 2019; 10: 352–357
Clinical Trial Registry
Japanese Clinical Trials Registry
UMIN000026780
References
- 1. Stratton IM, Adler AI, Neil HAW, et al Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000; 321: 405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ray KK, Seshasai SR, Wijesuriya S, et al Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta‐analysis of randomised controlled trials. Lancet 2009; 373: 1765–1772. [DOI] [PubMed] [Google Scholar]
- 3. Boussageon R, Bejan‐Angoulvant T, Saadatian‐Elahi M, et al Effect of intensive glucose lowering treatment on all cause mortality, cardiovascular death, and microvascular events in type 2 diabetes: meta‐analysis of randomised controlled trials. BMJ 2011; 343: d4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goto A, Arah OA, Goto M, et al Severe hypoglycaemia and cardiovascular disease: systematic review and meta‐analysis with bias analysis. BMJ 2013; 347: f4533. [DOI] [PubMed] [Google Scholar]
- 5. Miller ME, Williamson JD, Gerstein HC, et al Effects of randomization to intensive glucose control on adverse events, cardiovascular disease, and mortality in older versus younger adults in the ACCORD Trial. Diabetes Care 2014; 37: 634–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leiter L, Yale J‐F, Chiasson J‐L, et al Assessment of the impact of fear of hypoglycemic episodes on glycemic and hypoglycemia management. Can J Diabetes 2005; 29: 1–7. [Google Scholar]
- 7. Peyrot M, Barnett AH, Meneghini LF, et al Insulin adherence behaviours and barriers in the multinational Global Attitudes of Patients and Physicians in Insulin Therapy study. Diabetes Med 2012; 29: 682–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Trento M, Trevisan M, Coppo E, et al Diagnosis of type 1 diabetes within the first five years of life influences quality of life and risk of severe hypoglycemia in adulthood. Acta Diabetol 2014; 51: 509–511. [DOI] [PubMed] [Google Scholar]
- 9. Cooke D, O'Hara MC, Beinart N, et al Linguistic and psychometric validation of the Diabetes‐Specific Quality‐of‐Life Scale in U.K. English for adults with type 1 diabetes. Diabetes Care 2013; 36: 1117–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Strong J, Kruger D, Novak L. Insulin glargine 300 units/mL: a guide for healthcare professionals involved in the management of diabetes. Curr Med Res Opin 2017; 33: 785–793. [DOI] [PubMed] [Google Scholar]
- 11. Ritzel R, Roussel R, Bolli GB, et al Patient‐level meta‐analysis of the EDITION 1, 2 and 3 studies: glycaemic control and hypoglycaemia with new insulin glargine 300 U/ml versus glargine 100 U/ml in people with type 2 diabetes. Diabetes Obes Metab 2015; 17: 859–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ratner RE, Gough SC, Mathieu C, et al Hypoglycaemia risk with insulin degludec compared with insulin glargine in type 2 and type 1 diabetes: a pre‐planned meta‐analysis of phase 3 trials. Diabetes Obes Metab 2013; 15: 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marso SP, McGuire DK, Zinman B, et al Efficacy and safety of degludec versus glargine in type 2 diabetes. N Engl J Med 2017; 377: 723–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. International Hypoglycemia study Group . Glucose concentrations of less than 3.0 mmol/L(54 mg/dL) should be reported in clinical trials: a joint position statement of the American Diabetes Association and the European Association for the study of Diabetes. Diabetes Care 2017; 40: 155–157. [DOI] [PubMed] [Google Scholar]
- 15. Japanese Diabetes Society . 2018‐2019 Diabetes treatment guide, Bunkoudo, 2018: p. 29 (Japanese).
- 16. Becker RH, Dahmen R, Bergmann K, et al New insulin glargine 300 units·mL−1 provides a more even activity profile and prolonged glycemic control at steady state compared with insulin glargine 100 units·mL−1 . Diabetes Care 2015; 38: 637–643. [DOI] [PubMed] [Google Scholar]
- 17. Heise T, Hermanski L, Nosek L, et al Insulin degludec: four times lower pharmacodynamic variability than insulin glargine under steady‐state conditions in type 1 diabetes. Diabetes Obes Metab 2012; 14: 859–864. [DOI] [PubMed] [Google Scholar]
- 18. Kobuke K, Yoneda M, Nakanishi S, et al Efficacy and safety of insulin degludec in Japanese patients with type 1 and type 2 diabetes: 24‐week results from the observational study in routine clinical practice. J Diabetes Investig 2016; 7: 94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yki‐Jarvinen H, Bergenstal R, Ziemen M, et al New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using oral agents and basal insulin: glucose control and hypoglycemia in a 6‐month randomized controlled trial (EDITION 2). Diabetes Care 2014; 37: 3235–3243. [DOI] [PubMed] [Google Scholar]
- 20. Zinman B, Philis‐Tsimikas A, Cariou B, et al Insulin degludec versus insulin glargine in insulin‐naive patients with type 2 diabetes: a 1‐year, randomized, treat‐to‐target trial (BEGIN Once Long). Diabetes Care 2012; 35: 2464–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Riddle MC, Bolli GB, Ziemen M, et al New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using basal and mealtime insulin: glucose control and hypoglycemia in a 6‐month randomized controlled trial (EDITION 1). Diabetes Care 2014; 37: 2755–2762. [DOI] [PubMed] [Google Scholar]
- 22. Wysham C, Bhargava A, Chaykin L, et al Effect of insulin degludec vs insulin glargine U100 on hypoglycemia in patientswith type 2 diabetes: the SWITCH 2 randomized clinical trial. JAMA 2017; 318: 45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Garber AJ, King AB, Del Prato S, et al Insulin degludec, an ultra‐longacting basal insulin, versus insulin glargine in basal‐bolus treatment with mealtime insulin aspart in type 2 diabetes (BEGIN Basal‐Bolus Type 2): a phase 3, randomised, open‐label, treat‐to‐target non‐inferiority trial. Lancet 2012; 379: 1498–1507. [DOI] [PubMed] [Google Scholar]
- 24. Jonassen IB, Havelund S, Hoeg‐Jensen T, et al Design of the novel protraction mechanism of insulindegludec, an ultra‐long‐acting basal insulin. Pharm Res 2012; 29: 2104–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kramer W. New approaches to the treatment of diabetes. Exp Clin Endocrinol Diabetes 1999; 107: S52–S61. [Google Scholar]
- 26. Standl E, Owen DR. New long‐acting basal insulins: does benefit outweigh cost? Diabetes Care 2016; 39: S172–S179. [DOI] [PubMed] [Google Scholar]
- 27. Haahr H, Heise T. A review of the pharmacological properties of insulin degludec and their clinical relevance. Clin Pharmacokinet 2014; 53: 787–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bailey TS, Pettus J, Roussel R, et al Morning administration of 0.4 U/kg/day insulin glargine 300 U/mL provides less fluctuating 24‐hour pharmacodynamics and more even pharmacokinetic profiles compared with insulin degludec 100 U/mL in type 1 diabetes. Diabetes Metab 2018; 44: 15–21. [DOI] [PubMed] [Google Scholar]
- 29. Lee AJ. Metformin in noninsulin‐dependent diabetes mellitus. Pharmacotherapy 1996; 16: 327–351. [PubMed] [Google Scholar]
- 30. Song S, Andrikopoulos S, Filippis C, et al Mechanism of fat‐induced hepatic gluconeogenesis: effect of metformin. Am J Physiol Endocrinol Metab 2001; 281: E275–E282. [DOI] [PubMed] [Google Scholar]
- 31. McNally PG, Dean JD, Morris AD, et al Using continuous glucose monitoring to measure the frequency of low glucose values whenusing biphasic insulin aspart 30 compared with biphasic human insulin 30: a double‐blind crossover study in individuals with type 2 diabetes. Diabetes Care 2007; 30: 1044–1048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1¦ Flow diagram of study participants. The numbers in parentheses indicate the numbers of study participants at each step.


