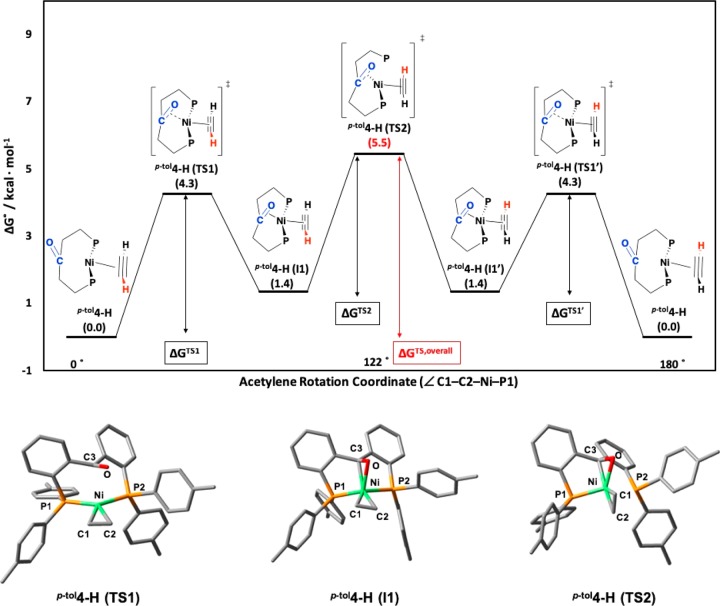
Figure 6.
(top) Energy diagram for the rotation of acetylene around the Ni-coordination plane (∠C1–C2–Ni–P1) of p-tol4-H through two different transition states, p-tol4-H(TS1) = p-tol4-H(TS1′) and p-tol4-H(TS2), and one intermediate, p-tol4-H(I1) = p-tol4-H(1′). Numbers in parentheses are G° values given in kcal/mol. ΔGTS1 = 4.3 kcal/mol. ΔGTS1′ = 2.9 kcal/mol. ΔGTS2 = 4.1 kcal/mol. ΔGTS,overall = 5.5 kcal/mol. (bottom) Optimized geometry of transition states p-tol4-H(TS1) and p-tol4-H(TS2) as well as intermediate p-tol4-H(I1) at a B3LYP/6-31g(d,p) level of theory under vacuum. The imaginary frequency (−113 cm–1) of p-tol4-H(TS1) shows the coordination of the ketone (C3–O) to Ni, while the imaginary frequency (−95 cm–1) of p-tol4-H(TS2) is the rotation of acetylene around the C1–C2–Ni–P1 torsion angle. Selected bond lengths [Å] for p-tol4-H(TS1): C3–O = 1.25; Ni–C3 = 2.35; Ni–O = 2.55; Ni–P1 = 2.17; Ni–P2 = 2.19; C1–C2 = 1.27. Selected bond lengths [Å] for p-tol4-H(I1): C3–O = 1.30; Ni–C3 = 2.01; Ni–O = 2.06; Ni–P1 = 2.18; Ni–P2 = 2.24; C1–C2 = 1.25. Selected bond lengths [Å] for p-tol4-H(TS2): C3–O = 1.32; Ni–C3 = 1.96; Ni–O = 1.92; Ni–P1 = 2.14; Ni–P2 = 2.45; C1–C2 = 1.24. Hydrogen atoms have been omitted for clarity.